INTRODUCTION
The tobacco epidemic is a major public health threat and one of the most important risk factors for premature deaths globally1. Tobacco kills more than 8 million people every year as a result of direct tobacco use, with another 1.2 million people dying as a result of secondhand smoke exposure2. Low- and middle-income countries, where the burden of tobacco related illness and death is maximum, account for more than 80% of the world’s 1.3 billion tobacco consumers3.
Oral cancer is the eighth most common cancer-related cause of death. Oral cancer claims the lives of over 175000 people each year, and over 370000 people are diagnosed yearly with the disease4. It is the leading form of cancer among males in South-East Asia and fourth most common form among the females5. Oral cancer is a widely prevalent cancer in developing countries and less prevalent in developed countries. However, a reversal in the down trend is being observed in developed countries4-7. The incidence of oral cancer in the United Kingdom has risen by 68% in the last 20 years8.
Oral squamous cell carcinoma is a type of oral cancer that accounts for more than 90% of all oral cancers9. Malignant neoplasm arising in the lining mucosa of the lips, mouth, including anterior two-thirds of tongue, is termed oral cancer. This case definition is adopted from World Health Organization case definition and International Agency for Research and Cancer, which conforms to the definition of oral cavity cancers by International Classification of Diseases (ICD)10. Oral potentially malignant disorders (OPMDs) are a category of lesions and conditions that are associated with a greater risk of developing cancers of the lip and oral cavity11. WHO classification on head and neck tumors has endorsed the terminology12.
Tobacco use is one of the major factors leading to the increasing rate of oral cancer4-8. Tobacco is classified into two groups depending on how it is consumed: smoked tobacco and smokeless tobacco. Smoking is the most common form of tobacco used worldwide and is highly addictive. Smokeless tobacco is chewed, snuffed or dipped rather than burned. The tobacco is cut up and moistened before chewing or keeping it between the gum and the cheek in the mouth. Herbs, areca nut, betel leaf, and slaked lime are some of the ingredients used with chewing tobacco. Smokeless tobacco products contain potentially harmful ingredients including tobacco-specific nitrosamines, cadmium, and nicotine. Tobacco is harmful in all forms, and there is no safe level of tobacco exposure. Smoking has been associated with oral cancers in different systematic reviews13,14. A significant association has also been noted between use of smokeless tobacco and oral cancer in systematic reviews at global level and region level, e.g. South-East Asia, Middle East, North Africa, Europe, and North America15-18.
Numerous studies have been conducted to associate tobacco with oral mucosal lesions. However, only a few evaluated and compared associations between smoking and chewing forms of tobacco with oral cancer and oral potentially malignant disorders19-32. This review provides a meta-analysis of the research evidence on the association between chewing and smoking use of tobacco with oral cancer and oral potentially malignant disorders. The research aim was to evaluate differences, if any, between tobacco smokers and chewers for oral cancer and oral potentially malignant disorders. The results will fill the gap in data on the independent effects of smoked and chewed tobacco on oral mucosa. The specific aim of the study was to compare, evaluate and quantify the pooled differences of the association between chewing and smoking forms of tobacco with oral malignancy or oral potentially malignant disorders.
METHODS
Inclusion criteria
Inclusion and exclusion criteria were determined prior to the literature search. Type of studies considered were all observational studies including prospective, retrospective, case control and cross-sectional studies evaluating the association between oral malignant and oral potentially malignant disorders with smoking and chewing forms of tobacco. Participants were adolescent children or adults with no age restrictions. Exposed to the risk factors were considered those participants with a habit of tobacco chewing and/or smoking. There were two mixed usage groups. Mixed usage smokers, this included only smokers and smokers who also chewed tobacco, and mixed usage chewers which included only chewers and tobacco chewers who also smoked. Consumption of either only tobacco chewing or only smoking was measured as non-mixed usage. The outcomes were oral malignancy or oral disorders with malignant potential.
Assessment of oral cancer and other potentially malignant disorders was made clinically using the International Statistical Classification of Diseases and Related Health Problems, 10th version, ICD-10, with a histological confirmation. Any malignant neoplasm arising in the lining mucosa of the lips, mouth, including anterior two-thirds of the tongue, was termed as oral cancer as defined in the ICD cancer diagnostic groups. Posterior third of tongue and oropharyngeal sites were not included.
Exposure was assessed using history/questionnaire assessments at the time of clinical examination. Current tobacco smokers or chewers were daily users of tobacco for at least one year irrespective of the level of consumption. Former tobacco users were defined as those who used either smoking or chewing tobacco but currently do not use any form of tobacco. Adults who reported that they neither smoked nor used smokeless tobacco in their life time were defined as never users. Occasional users, former users, or daily users exposed for less than one year were not considered in the meta-analysis.
Search strategy
A search was conducted in Medline, Cochrane databases, Google Scholar, Scopus and Web of Science for studies up to December 2021. PRISMA guidelines were followed for the meta-analysis. Keywords used for conducting literature search were: precancerous conditions, oral cancer, chewing tobacco, smokeless tobacco, tobacco smoking. Boolean operators used with keywords are given in the Supplementary file. Selection of studies was independently carried out by the two investigators (AS and BP). First, a pre-screening of title and abstracts was conducted to decide the studies to be retrieved in full and to exclude ineligible studies. Second, the retrieved studies were examined to be select those to be included in the review. Differences between the two investigators were resolved by discussion. A third person with subject expertise had been pre-approved by the two investigators if the need arose, if there was a lack of consensus. References in the selected articles were manually reviewed and retrieved if they were possibly relevant. The articles were searched using English keywords. However, no restrictions were placed on language of publication. Attempt was also made to search grey literature through unpublished articles and manual searching of non-indexed journals, but no articles were retrieved. The grey literature was searched in non-indexed journals at the library of the All India Institute of Medical Sciences, New Delhi, (AIIMS) New Delhi and AIIMS, Bhopal. Grey literature was also searched through abstracts, presentations at conferences, online clinical registries including results of completed but unpublished trials, for inclusion in the review. The study protocol was registered with PROSPERO at Centre for Reviews and Dissemination, University of York (CRD42021242894).
Data extraction and quality assessment
Outcomes data were extracted independently in a pilot tested worksheet by the two investigators (AS and BP) using guidelines by the Cochrane Collaboration. Differences between the two investigators were resolved by discussion. Characteristics of the studies included in meta-analysis are presented in Table 1. Quality was evaluated by both the investigators using the Risk of Bias Assessment tool for Non-randomized Studies14. Six domains, i.e. participant selection, confounding variables, exposure measurement, attrition for prospective studies, incomplete outcome date, and selective outcome reporting, were evaluated according to low, moderate, and high risk of bias (Table 2). Moderate risk of bias was allotted to studies where randomization was not stated explicitly. Attrition was reported only for prospective studies. Heterogeneity in meta-analysis is the difference in study outcomes among the included studies. The I2 statistic denotes the percentage difference in study results that are due to heterogeneity rather than chance. A value of 0% represents no observed heterogeneity, and bigger values reflect increasing heterogeneity. A decline in the I2 value implies that the heterogeneity was being contributed by studies which were not part of this group.
Table 1
Characteristics of studies included in the meta-analysis and number of participants with precancerous and cancerous lesions, according to tobacco use
| Authors Date Location | Study design and duration | Number of participants | Age group (years) | Tobacco smokers | Tobacco chewers | Mixed users (smoking + chewing) |
|---|---|---|---|---|---|---|
| Aishwarya et al.19 2018 India | Cross-sectional 4 months (2015) | Sample size: 280 Tobacco smokers: 50 Tobacco chewers: 50 Mixed: 40 | ≥18 | Precancerous lesions: 21 Oral cancer: 1 | Precancerous lesions: 36 Oral cancer: 7 | Precancerous lesions: 22 Oral cancer: 4 |
| Behura et al.20 2015 India | Case control 12 months (2013) | Sample size: 450 Cases: 150 (with lesions) Control: 300 (without lesions) Tobacco smokers: 55 Tobacco chewers: 55 Mixed: 40 | ≥15 | Precancerous lesions: 19 Oral cancer: 1 | Precancerous lesions: 34 Oral cancer: 8 | Precancerous lesions: 21 Oral cancer: 5 |
| Gupta et al.21 2014 India | Cross-sectional 4 years (2006–2009) | Sample size: 471 (from 147983 screened patients) Tobacco smokers: 72 Tobacco chewers: 116 Mixed: 102 | ≥18 | Precancerous lesions: 22 Oral cancer: 9 | Precancerous lesions: 64 Oral cancer: 24 | Precancerous lesions: 70 Oral cancer: 10 |
| Joshi et al.22 2016 India | Case control 12 months (2013) | Sample size: 4795 Cases: 150 Control: 300 Tobacco smokers: 1748 Tobacco chewers: 1819 Mixed: Not reported | ≥15 | Precancerous lesions: 1628 | Precancerous lesions: 1590 | Not reported |
| Kavarodi et al.23 2014 Qatar | Cross-sectional 5 years (2005–2010) | Sample size: 1375 (from 3946 screened patients) Tobacco smokers: 958 Tobacco chewers: 169 Mixed: 248 | ≥18 | Precancerous lesions: 30 | Precancerous lesions: 15 | Precancerous lesions: 30 |
| Koothati et al.24 2020 Peru | Cross-sectional 6 months (2012) | Sample size: 3200 Tobacco smokers: 320 Tobacco chewers: 334 Mixed: 248 | 16–75 | Precancerous lesions: 12 | Precancerous lesions: 52 | Precancerous lesions: 30 |
| Priya et al.25 2018 India | Cross-sectional 3 months (2011) | Sample size: 300 Tobacco smokers: 148 Tobacco chewers: 101 Mixed: 51 | ≥15 | Precancerous lesions: 12 | Precancerous lesions: 16 | Precancerous lesions: 4 |
| Sujatha et al.26 2012 India | Cross-sectional 9 months (2010–2011) | Sample size: 1028 Tobacco smokers: 403 Tobacco chewers: 289 Mixed: 226 | Not reported | Precancerous lesions: 108 Oral cancer: 1 | Precancerous lesions: 142 Oral cancer: 5 | Precancerous lesions: 95 Oral cancer: 2 |
| Vikneshan et al.27 2016 India | Cross-sectional 9 months (2010–2011) | Sample size: 1500 Tobacco smokers: 245 Tobacco chewers: 950 Mixed: 305 | Industrial workers (age group not reported) | Precancerous lesions: 46 Oral cancer: 0 | Precancerous lesions: 251 Oral cancer: 4 | Precancerous lesions: 14 Oral cancer: 4 |
| Vinay et al.28 2014 India | Cross-sectional 3 months (2012) | Sample size: 1200 Tobacco smokers: 136 Tobacco chewers: 150 Mixed: Not reported | 20–70 | Precancerous lesions: 16 Oral cancer: 0 | Precancerous lesions: 29 Oral cancer: 0 | Not reported |
| Amtha et al.29 2015 Indonesia | Case control 1 year and 4 months (2005–2006) | Sample size: 280 Cases: 81 Control: 162 Tobacco smokers: 124 Tobacco chewers: 9 Mixed: 0 | 23–74 | Oral cancer: 45 | Oral cancer: 6 | Oral cancer: 0 |
| Lin et al.30 2010 Taiwan | Cohort 2 years and 8 months (2005–2008) | Sample size: 10657 Tobacco smokers: 2268 Tobacco chewers: 758 Mixed: 520 | ≥18 | Oral cancer: 174 | Oral cancer: 126 | Not reported |
| Madani et al.31 2020 India | Case control 1 year and 7 months (2005–2006) | Sample size: 700 Cases: 350 Control: 350 Tobacco smokers: 186 Tobacco chewers: 225 Mixed: Not reported | ≥18 | Oral cancer: 125 | Oral cancer: 175 | Not reported |
| Pednekar et al.32 2011 India | Cohort Recruitment: 1991–1997 Follow up: 5.5 years (1997–2003) | Sample size: 88658 Follow up: 87222 Tobacco smokers: 27361 Tobacco chewers: 33682 Mixed: Not reported | ≥35 | Oral cancer: 518 | Oral cancer: 476 | Not reported |
Table 2
Quality assessment of studies included in the meta-analysis
| Study | Selection of participants | Confounding variables | Measurement of exposure | Attrition (prospective studies only) | Incomplete outcome data | Selective outcome reporting |
|---|---|---|---|---|---|---|
| Aishwarya et al.19 2018 | L | L | L | NA | L | L |
| Behura et al.20 2015 | L | L | L | NA | L | L |
| Gupta et al.21 2014 | L | M | L | NA | L | L |
| Joshi et al.22 2016 | L | M | L | NA | M | M |
| Kavarodi et al.23 2014 | L | M | L | NA | L | L |
| Koothati et al.24 2020 | L | L | L | NA | L | L |
| Priya et al.25 2018 | L | M | L | NA | L | L |
| Sujatha et al.26 2012 | L | L | L | NA | L | L |
| Vikneshan et al.27 2016 | L | L | L | NA | L | L |
| Vinay et al.28 2014 | L | M | L | NA | M | M |
| Amtha et al.29 2015 | L | M | L | NA | L | L |
| Lin et al.30 2010 | L | L | L | NA | L | L |
| Madani et al.31 2020 | L | L | L | NA | M | M |
| Pednekar et al.32 2011 | L | M | L | L | M | M |
Statistical analysis
Systematic review was conducted using Cochrane Program Review Manager Version 5. Random effects model was used to pool and compare the association between smoking and chewing forms of tobacco with oral malignancy or oral potentially malignant disorders. Mantel-Haenszel estimate was used in the Random effects model. Summary odds ratio with 95% confidence interval was calculated for evaluating the association levels. Assessment of certainty of evidence was conducted using GRADE analysis. Statistical significance was set at p<0.05.
RESULTS
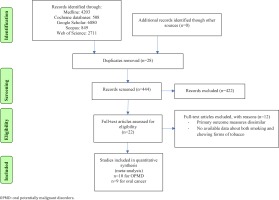
Literature search yielded 22 potentially relevant publications, of which 14 were included in the review as shown in Figure 1. Ten studies were included for the meta-analysis which assessed the association between chewing and smoking forms of tobacco with pre-malignant disorders. Similarly, nine studies were included which assessed association between chewing and smoking forms of tobacco with oral cancers. Characteristics of the included studies are described in Table 1. A total of 30764 tobacco smokers and 36134 tobacco chewers with mixed usage, were available for analysis for oral cancers. Similarly, 4135 tobacco smokers and 4033 tobacco chewers with mixed usage, were available for analysis for oral potentially malignant disorders. Majority of the studies included were cross-sectional in nature and involved Asian populations. Cigarettes and bidis were the most commonly used forms of smoked tobacco. The most commonly involved sites for oral cancer and other potentially malignant disorders were buccal mucosa and lateral surface of tongue.
Table 2 presents the quality of studies included in the analysis. A low risk of bias was present under the domain for selection of participants. Attrition levels were low in prospective studies. Also, a low to moderate risk of bias was present for incomplete outcome data and selective outcome reporting.
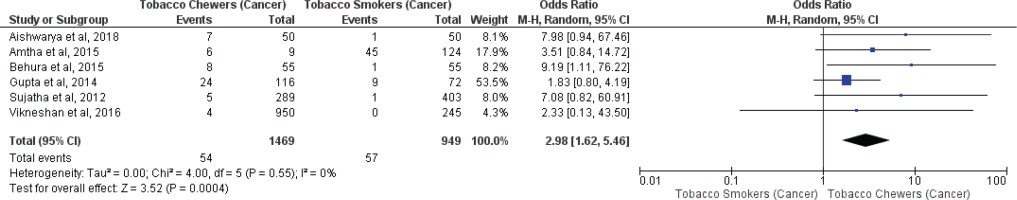
Meta-analysis of studies evaluating presence of oral potentially malignant disorders associated with tobacco smokers and chewers for mixed (overall) and no mixed usage are presented in Figures 2 and 3. Tobacco chewers were significantly associated with higher presence of oral potentially malignant disorders among patients with both mixed (OR=2.20; 95% CI: 1.25–3.87, p=0.006; I2=93%) and no mixed usage (OR=2.63; 95% CI: 2.01–3.43, p=0.001; I2=44%).
Figure 2
Oral potentially malignant disorders associated with smoking (only smokers + smokers who also chew tobacco) and chewing (only chewers + chewers who also smoke) forms of tobacco
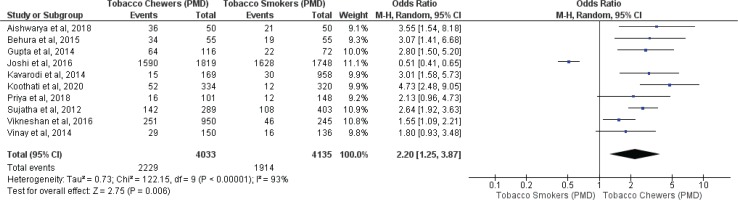
Figure 3
Oral potentially malignant disorders associated with smoking and chewing forms of tobacco with no mixed usage
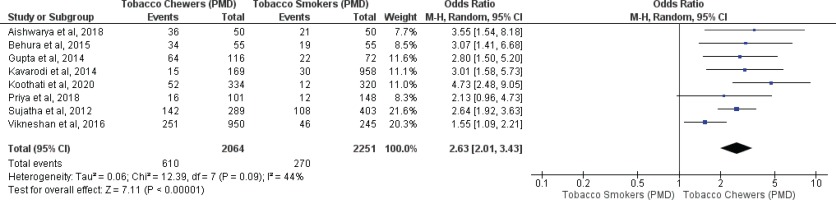
Meta-analysis of studies evaluating oral malignancy associated with tobacco smokers and chewers for mixed (overall) and no mixed usage are presented in Figures 4 and 5. Tobacco chewers were significantly associated with the presence of higher oral malignancy among patients with both mixed (OR=2.26; 95% CI: 1.19–4.28, p=0.01; I2=91%) and no mixed usage (OR=2.98; 95% CI: 1.62–5.46, p=0.0001; I2=0%).
Figure 4
Oral malignancy associated with smoking (only smokers + smokers who also chew tobacco) and chewing (only chewers + chewers who also smoke) forms of tobacco
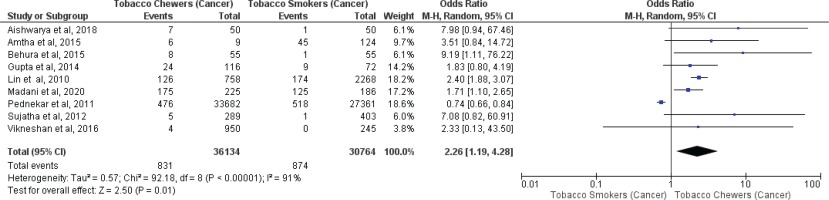
Differences in I2 values from 93% to 44% were observed among studies conducted on only tobacco chewers, and smokers with no mixed consumption, for association with oral potentially malignant disorders. Similarly, differences in I2 values were noted from 91% to 0% were observed among studies conducted on only tobacco chewers, and smokers with no mixed usage, for association with oral malignancy.
The level of evidence obtained from all observational studies for association between smoking and chewing forms of tobacco with oral potentially malignant lesions including mixed and non-mixed usage was moderate. Similarly, a moderate level of evidence was noted for the association between oral malignancies with tobacco usage (Table 3). Funnel plots were drawn for studies included in the pooled analysis for evaluating differences in association between tobacco chewers and tobacco smokers for oral potentially malignant disorders and oral malignancy (Figure 6).
Table 3
GRADE assessment for certainty of evidence for association between chewing and smoking forms of tobacco with oral potentially malignant disorders and oral malignancy with mixed and no mixed tobacco usage
| Certainty assessment | Number of patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Study design | Risk of bias | Inconsistency | Indirectness | Impression | Other considerations | Tobacco smoker | Tobacco chewer | OR 95% CI | Certainty |
| Association between smoking and chewing forms of tobacco with oral potentially malignant lesions (mixed usage) | ||||||||||
| 10 | Observational | Not serious | Serious | Not serious | Not serious | Strong association* | 1914/4135 (46.3%) | 2229/4033 (55.3%) | 0.45 0.26–0.80 | ⊕⊕⊕◯ Moderate |
| Association between smoking and chewing forms of tobacco with oral potentially malignant lesions (no mixed usage) | ||||||||||
| 8 | Observational | Not serious | Not serious | Not serious | Not serious | Strong association* | 270/2251 (12%) | 610/2064 (29.6%) | 0.38 0.29–0.50 | ⊕⊕⊕◯ Moderate |
| Association between smoking and chewing forms of tobacco with oral cancer (mixed usage) | ||||||||||
| 9 | Observational | Not serious | Serious | Not serious | Not serious | Strong association* | 874/30764 (2.8%) | 831/36134 (2.3%) | 0.44 0.23–0.84 | ⊕⊕⊕◯ Moderate |
| Association between smoking and chewing forms of tobacco with oral cancer (no mixed usage) | ||||||||||
| 6 | Observational | Not serious | Not serious | Not serious | Not serious | Strong association* | 57/949 (6%) | 54/1469 (3.7%) | 0.34 0.18–0.62 | ⊕⊕⊕◯ Moderate |
DISCUSSION
This meta-analysis is the first in scientific literature to evaluate and assess the differences in association between tobacco smokers and chewers with oral cancer or oral potentially malignant disorders. Our meta-analysis demonstrated a significant higher association for tobacco chewers with oral cancer and oral potentially malignant disorders, when compared with tobacco smokers.
It is essential to assess consistency of effects across studies in a meta-analysis. Differences in I2 values were noted among studies conducted on only tobacco chewers and smokers with no mixed usage, for association with oral malignancy or oral potentially malignant disorders. A lower heterogeneity is desirable as it reflects consistent finding across studies. Quality of evidence, as evaluated by GRADE, was moderate for the strong association between tobacco chewing with oral malignancy and oral potentially malignant disorders, with no mixed usage. Level of evidence was moderate for the overall association between tobacco chewing with oral malignancy or oral potentially malignant disorders, due to inconsistency in results.
Tobacco use has major economic costs, which include significant healthcare costs for treating diseases caused by tobacco use, as well as lost human resources due to tobacco-related morbidity and mortality. Tobacco use accounts for more than 80% of oral cancers and is a major cause of lost productivity due to premature deaths. With over 350 million users worldwide, smokeless tobacco use is a serious public health challenge, particularly in South-East Asia33. Users of both chewing and smoking products realize tobacco cessation is more challenging to achieve than among those who only chew or smoke tobacco34. A need for research on curbing major challenges in regulating use of smokeless tobacco has been long felt and subsequently highlighted in several sessions of the Framework Convention on Tobacco Control (FCTC)35.
Combating the tobacco epidemic is enormous and among the gravest public health challenges. Smokeless tobacco use is increasing rapidly in many regions including South-East Asia, especially among young people36. A misperception that exists is that using tobacco products like smokeless tobacco is less detrimental to one’s health than smoking cigarettes. In most countries, there are no health warnings on the packaging of smokeless tobacco34,35. This adds to the idea that using chewing tobacco is relatively risk-free. This study establishes that although tobacco consumption is hazardous in any form, chewing tobacco is more injurious due to higher association with oral malignancy and other malignant disorders, compared to smoked tobacco.
Limitations
Individual effects of tobacco and areca were not separated and could be considered as study limitation. Sub-group analysis and sensitivity analysis were not considered with the high heterogeneity levels in the study. Also, the majority of the included studies were cross-sectional in nature. These are few of the study limitation. However, this study establishes that chewing tobacco is not a safe alternative to smoking. The present study was an attempt to fill the knowledge gap and to provide evidence for comparative association between chewed and smoked tobacco with oral malignancy and oral potentially malignant disorders.
CONCLUSIONS
Our meta-analysis pools evidence that consumption of chewing tobacco is significantly associated with higher oral malignancy and oral potentially malignant disorders, compared to smoking tobacco. Further prospective studies need to be conducted to confirm this association between chewing and smoking forms of tobacco with oral malignancy and oral potentially malignant disorders, due to a moderate certainty of evidence presented in our study.