INTRODUCTION
India has the second largest number of tobacco users in the world1. Tobacco use accounts for over 1.3 million deaths a year in the country2,3 and the youth remain among the most vulnerable population segments in terms of tobacco-related harms. It is at this stage in life that people usually experiment and/or initiate tobacco use. In India, the average age at which people take up tobacco use is 18.9 years4. Tobacco use among youth in India is quite high: 14.6% users in the age group 13–15 years in the year 20095, and 12.4% users in the age group 15–24 years in the years 2016–20176. Tobacco is also used in diverse forms. Analysis of a nationally representative survey done in 2009 revealed that among youth tobacco users, 35.1% smoked cigarettes or bidis and 43.3% used chewing or smokeless tobacco, while 21.5% youth used more than one form of tobacco7.
Along with tobacco use, use of supari (areca nut) in various forms, included as an ingredient in smokeless tobacco products, is common in India and is linked to various health hazards including oral cancers8. Studies among youth in Mumbai (India) report the prevalence of ever use of supari products to be from about 18% to about 32%9,10. Adding to their vulnerability, studies have shown that the tobacco industry specifically targets youth through their marketing strategies11. Studies have shown an association between youth uptake of tobacco use and having parents, family members and peers using tobacco, exposure to tobacco portrayal in media, undergoing stressful episodes, and access to disposable pocket money12,13.
Schools provide an excellent setting for health promotion interventions in engaging youth and related stakeholders14. While there have been regulatory measures (e.g. ban on sale of tobacco to minors and around educational institutions15, and educational interventions such as school-based programs as part of the National Tobacco Control Program16), there is a dearth of school-based interventions offering support to youth to quit tobacco. This is despite a huge need: nearly two-thirds of the student smokers expressed desire to quit smoking in a national survey5. In fact, offering cessation support to current users is recognized as an important strategy by the World Health Organization in its MPOWER strategy package for tobacco control17. While documented interventions in this domain in India are themselves limited in number, they are rarely evaluated for their impact. At present, there is limited evidence available in the form of two different interventions, one for school teachers in Bihar (2009–2011)18 and another for school students (6th and 8th grades) from Delhi and Chennai cities (2004–2006)19, which provided positive evidence for potential of schoolbased tobacco control interventions in reducing tobacco use. Furthermore, systematic reviews of the studies around smoking or tobacco cessation have pointed to the positive impact of group-based counselling sessions20,21.
It is in this context of very limited evidence around school-based tobacco cessation interventions that we aimed to assess the impact of the LifeFirst program. LifeFirst is an ongoing tobacco and supari cessation intervention delivered to the students from low socioeconomic stratum studying in corporation (local government) schools in Mumbai city22. The specific objectives of our study conducted in intervention and control schools in Mumbai were to assess and compare the change in: 1) prevalence of tobacco and supari use, 2) knowledge about harms of tobacco and supari, and 3) perceived skills to refuse tobacco and supari among the students receiving and not receiving the LifeFirst tobacco and supari cessation intervention in school.
METHODS
Study design
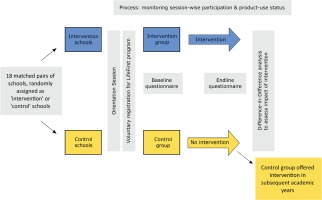
We used a prospective quasi-experimental design with an intervention and a control arm embedded within an ongoing LifeFirst tobacco and supari cessation program. The participants of the program were students who wished to quit their tobacco and supari usage. The students assigned to the intervention group/schools registered and received LifeFirst program intervention while those assigned to the control group/schools were registered for the LifeFirst program but were to receive the program intervention in the subsequent academic year. Hence, the latter group served as a natural control. A Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) statement checklist provides the details of the various study components and their location in the manuscript (See Supplementary File)23.
We used a difference-in-difference analysis24 with baseline and end-line questionnaires to assess the program’s impact on students’ knowledge about tobacco/supari harms, students’ refusal skills with regard to tobacco/supari use, and the prevalence of tobacco/supari use. Figure 1 depicts the overall study design.
About the LifeFirst intervention
The LifeFirst is a standardized tobacco and supari cessation program conceived and implemented by the Narotam Sekhsaria Foundation, India, since 2012. The Program targets students from 7th to 9th grades in corporation (local government) funded/managed schools in Mumbai city. The typical program cycle runs through a given academic year and is delivered by trained program staff in the selected schools in a separate space allocated by the school authorities. It starts with an orientation program in select schools where students are sensitized about harms of tobacco and supari use. Following the orientation session, students using tobacco and/or supari are invited to register at their will (ie. voluntary registration) and be part of the main program, which includes delivery of six group-based education and counselling sessions by trained counsellors at a designated location within their respective schools. These interactive sessions are of 40–50 minutes duration conducted at monthly intervals and focus on specific themes (i.e. group bonding, tobacco harms, barriers, refusal skills). Intervention sessions focused on understanding the types of tobacco and supari products, associated health harms, ways of quitting, the concept of craving, temptation, withdrawal and coping mechanisms, and relapse. Additionally, as a part of refusal skills, the sessions emphasized on differentiating between passive, assertive and aggressive behaviors and the importance of assertive behavior and had activities that used roleplay of refusal skills for tobacco and supari products. In order to maintain attendance and participation, the program intervention was conducted at the school campus within school hours, and program activities were designed to be diverse and engaging. A more detailed description is provided in the Supplementary file. The program keeps record of students’ participation and their tobacco/supari use status overtime.
Sample size
Our primary outcomes included the change in the proportion of tobacco users and supari users among registered students in the course of the program and comparing these measures across intervention and control groups. The closest relevant indicator we could use to estimate the required sample size for such a study came from the LifeFirst program data recorded for the earlier program cycles (2012–2013 to 2016–2017). On average, about 65% of registered students quit tobacco and/or supari use based on students’ self-report at the end of the respective program cycles. While this was a mixed measure (for tobacco and supari) and not exactly the one we aimed to use for our study, we believe it provided a valuable guide to estimate the sample size for our study. Using this value, we used STATA to estimate the sample size required to detect an effect size of ten percentage points (a difference between intervention and control groups) with 80% power and significance level (alpha) of 0.05. We added 10% to this number to compensate for any design defects arriving at a minimal sample size of about 436 students per group.
Recruitment of participants
Our study focused on the academic year June 2017 – March 2018 and the associated program cycle of the LifeFirst. We first chose the schools and then recruited students from those schools into intervention and control groups. The Municipal Corporation of Greater Mumbai represents the largest primary education system in India: about 1162 schools (approximately 394599 students) directly funded and managed by the corporation (often referred as corporation schools) and about 450 schools (approximately 300504 students) receiving financial aid from the corporation but being managed privately (often referred to as aided schools)25. Corporation schools provide free education while the aided schools charge nominal fees, primarily catering to, and at times the only option for, economically weaker sections of Mumbai residents25. While these schools have been facing challenges of declining enrollment rates and suboptimal learning outcomes, they do provide seamless education from pre-primary up to 10th standards with low dropout rates26.
We matched the schools for which the LifeFirst had received necessary permissions to engage with in the given academic year on four criteria: their location (municipal ward), the medium of instruction (Hindi, Urdu, English, Marathi), the school type (corporation schools, aided schools), and the grades they offered (7th, 8th, 9th). We arrived at 18 matching pairs of schools. Within matched pairs, we randomly allocated one to the intervention group and the other to the control group. The LifeFirst staff, blinded to the allocation of schools into intervention and control groups, conducted orientation sessions on tobacco/supari harms in these schools and started enrolling the willing students (i.e. the current users of tobacco and/or supari who were willing to stop their use) into the LifeFirst program following these sessions in their respective schools. A real-time inventory was kept concerning the number of students being enrolled from intervention and control schools. We kept including schools till we reached the estimated sample size.
Data collection
We used a self-administered questionnaire to collect data from the students enrolled into the LifeFirst program. We administered the baseline questionnaire as a part of the first session of the LifeFirst program (before the intervention) and the end-line questionnaire in the last session of the program (after the intervention). The questionnaires used in these two rounds were identical, except for a section in the end-line questionnaire assessing the students’ participation in and feedback on the LifeFirst program. The questionnaires broadly assessed: 1) personal and sociodemographic characteristics of students; 2) their knowledge, attitudes and practice concerning tobacco and supari use; 3) their quit attempts and outcomes; and 4) data on variables that are known to influence uptake of tobacco and supari (e.g. exposure to others using tobacco/supari, portrayal of tobacco/supari use in media, access to tobacco/supari products, and knowledge and perceived enforcement of tobacco control policies in schools). The questionnaires adapted elements from tools that have been validated and used previously in India for assessing tobacco use and related factors among youth. These included the core questionnaire used in Global Youth Tobacco Survey (India)27 and a questionnaire used among pre-university students in Karnataka28. The initial questionnaire developed in English was translated into Hindi, Marathi and Urdu, and was back translated into English to ensure accuracy in meaning.
LifeFirst staff, who undertook training for data collection for this study and were blinded to the intervention/control status of the schools, administered the questionnaires. The children participating in the study were also blinded to the intervention and control status and only the researchers evaluating the program were aware of the intervention and control group details. After securing consent, from the school authorities and parents of the children, data collectors provided instructions, to all the children in a separate space allocated by the school to conduct the LifeFirst related activities, distributed the questionnaires and assisted those students who had queries on any specific questions.
Measures
The primary outcome variables included point prevalence of tobacco use, and point prevalence of supari use, among students registered for LifeFirst program. The prevalence of tobacco and/or supari use was estimated based on the use of any products containing tobacco and/or supari in the 30 days preceding the questionnaire. The secondary outcome variables included knowledge score on tobacco harms and supari harms. These scores were arrived at by simple summation of scores for individual questions (1 for correct answer; 0 for incorrect answer) assessing knowledge on tobacco and supari harms. The tobacco knowledge score ranged from 0 to 6, while the supari knowledge score ranged from 0 to 2. We also included refusal scores for tobacco products among secondary outcomes, derived by simple summation of scores for individual questions (1 confirming refusal; 0 indicating non refusal) on students’ ability to refuse offers of tobacco from others, and ranged from 0 to 3.
We used a set of independent/exposure variables that are known to influence tobacco use uptake/status among youth. These included students’ age, sex, school grade, medium of instruction, and pocket money.
Statistical analysis
Trained professionals generated electronic data from the baseline and end-line surveys using Epidata Manager (v4.6.0.0)29. We used standard data entry forms with validity checks and manually verified 10% of the entered data using physical forms to ensure accuracy. We used Stata SE 15.130 to analyze data. We conducted a difference-in-difference analysis for the five outcome variables in the study. The difference-in-difference analysis compares the changes in outcome variables over time between the intervention and control group, keeping in consideration the initial differences between these two groups. We also adjusted such analysis using relevant covariates. Finally, to promote transparency and wider use, we also published our dataset on Figshare, a publicly accessible research and data repository31.
RESULTS
A total of 959 students volunteered to register in the LifeFirst program in order to quit their tobacco/supari use habit. From this, 827 students completed both the baseline and end-line questionnaires, yielding a response rate of 86.2% and these students formed our population for analysis. The other 132 students (66 each in the intervention and control group) were not included as they answered either the baseline or the end-line survey. The reasons for nonresponse included absenteeism on the day of the surveys and/or transfer to other schools.
Table 1 provides characteristics of the final sample population including the outcome measures at the baseline, reported by study condition along with indication of statistically significant differences between the two groups. On the whole, 17% of students used tobacco and 97% used supari in the 30 days preceding the baseline survey. Most of the tobacco users used supari as well, with the proportion of dual users being 15.7% in the control group and 16.8% in the intervention group. We found a greater degree of participation by students in all the six thematic sessions in the intervention group: the median number of sessions participated by students in the intervention group was 5.07 (SD=1.2). Our recruitment strategy of using matched schools yielded a largely matching sample. Both the groups were identical in their background characteristics except for the educational level, medium of instruction, and exposure to media advertising.
In terms of outcomes at the baseline, the students in the control group had marginally higher knowledge scores for tobacco and supari harms. However, our use of the difference-in-difference analysis accounted for these initial differences across the two groups. Table 2 presents the findings from the difference-in-difference analysis for each of the outcome variables.
Table 1
Major characteristics of the sample population at baseline
| Control (N=414) | Intervention (N=413) | |
|---|---|---|
| Sex, n (%) | ||
| Boys | 261 (63.0) | 270 (65.4) |
| Girls | 153 (37.0) | 143 (34.6) |
| Age (years), mean (SD) | 13.3 (1.3) | 13.4 (1.5) |
| Current education level, n (%)** | ||
| 7th grade | 107 (25.9) | 136 (33.0) |
| 8th grade | 144 (34.8) | 106 (25.7) |
| 9th grade | 163 (39.4) | 170 (41.3) |
| Medium of instruction, n (%)** | ||
| Marathi | 194 (46.9) | 198 (47.9) |
| Hindi | 105 (25.4) | 61 (14.8) |
| Urdu | 84 (20.3) | 131 (31.7) |
| English | 31 (7.5) | 23 (5.6) |
| Pocket money/week (INR), mean (SD) | 78.5 (82.5) | 83.6 (79.6) |
| Outcome variables at baseline | ||
| Tobacco use prevalence, n (%) | 67 (17.1) | 69 (17.7) |
| Supari use prevalence, n (%) | 402 (97.1) | 399 (96.1) |
| Dual user, n (%) | 62 (15.74) | 66 (16.88) |
| Age of initiation (years), mean (SD) | 11.3 (1.7) | 11.1 (2.1) |
| Tobacco knowledge score 0–6, mean (SD) ** | 4.6 (0.9) | 4.4 (1.1) |
| Supari knowledge score 0–2, mean (SD) ** | 1.7 (0.5) | 1.5 (0.6) |
| Refusal score 0–3, mean (SD) | 2.6 (0.8) | 2.7 (0.7) |
| Exposure to tobacco use by friends/family (Y), n (%) | 347 (83.8) | 350 (84.8) |
| Exposure to supari use by friends/family (Y), n (%) | 364 (87.9) | 369 (89.4) |
| Exposure to tobacco or supari use by school staff (Y), n (%) | 111 (56.4) | 147 (59.3) |
| Exposure to advertising in media (Y), n (%)** | 368 (90.4) | 343 (83.9) |
| Knowledge of school policy in action at own school (Y), n (%) | 336 (92.1) | 340 (95.0) |
Table 2
Difference-in-difference analysis for outcome variables
| Intervention (I) | Control (C) | Difference (I-C) | Unadjusted difference-indifferences estimator (SE) | Adjusted difference-indifferences estimatora (SE) | |
|---|---|---|---|---|---|
| Point prevalence of tobacco use (SE) | |||||
| Before (B) | 0.176 | 0.171 | 0.006 (0.028) | ||
| After (A) | 0.116 | 0.29 | -0.173 (0.027)** | ||
| Difference (A-B) | -0.179 (0.039)** | -0.175 (0.038)** | |||
| Point prevalence of supari use (SE) | |||||
| Before (B) | 0.966 | 0.971 | -0.005 (0.024) | ||
| After (A) | 0.232 | 0.618 | -0.386 (0.024)** | ||
| Difference (A-B) | -0.381 (0.034)** | -0.379 (0.034)** | |||
| Tobacco knowledge score (SE) (Range: 0-6) | |||||
| Before (B) | 4.375 | 4.572 | -0.197 (0.065)** | ||
| After (A) | 4.978 | 4.778 | 0.200 (0.065)** | ||
| Difference (A-B) | 0.398 (0.092)** | 0.406 (0.093)** | |||
| Supari knowledge score (SE) (Range: 0–2) | |||||
| Before (B) | 1.547 | 1.698 | -0.151 (0.035)** | ||
| After (A) | 1.831 | 1.751 | 0.079 (0.035)** | ||
| Difference (A-B) | 0.230 (0.049)** | 0.229 (0.05)** | |||
| Refusal score for tobacco products (SE) (Range: 0-3) | |||||
| Before (B) | 2.712 | 2.599 | 0.113 (0.047)** | ||
| After (A) | 2.814 | 2.688 | 0.125 (0.047)** | ||
| Difference (A-B) | 0.012 (0.067) | 0.018 (0.068) |
Tobacco and supari use
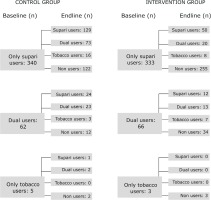
We hypothesized that the participation in the LifeFirst program would lead to reduction in the point prevalence of tobacco and supari use. There was no statistically significant difference in these outcome measures across intervention and control groups at the baseline. At the end of the intervention, tobacco use decreased in the intervention group (17.6% to 11.6%) while it increased in the control group (17.1% to 29%). The difference-indifference estimates, adjusted for relevant covariates, show statistically significant reduction of 17.9 percent points in the intervention group. The supari use decreased in both the groups but it decreased by a greater proportion among the intervention group compared to the control group with an adjusted difference-in-difference estimator suggesting a statistically significant reduction of 38.1 percent points in intervention beyond the reduction in the control group. The intervention not only led to significant reduction in tobacco and supari use but also had protective effects against new uptake of tobacco use: by the end of the study period, 89 supari users in the control group (out of 340) took up tobacco, whereas only 28 in the intervention group (out of 333) did the same (Figure 2).
Knowledge scores
Both the groups had high knowledge scores for tobacco harms at the baseline with the control group having a marginally greater tobacco knowledge score (4.57 out of 6) compared to the intervention group (4.37 out of 6). The same was true for the knowledge score about supari harms. While the knowledge scores improved for both the groups during the intervention period, it improved by a greater percentage among the intervention group compared to the control group; the adjusted difference-in-difference estimator showed a net increase of 0.41 and 0.21 in tobacco and supari knowledge scores among the intervention group. This difference was statistically significant.
Refusal score
Refusal score assessed the ability of students to refuse offers of uptake of tobacco from their near ones. We found that the refusal score significantly improved in both the groups during the intervention. While the improvement was greater among the intervention group compared to the control group, the net increase in the refusal score among the intervention group was very marginal (0.23) and was statistically insignificant.
DISCUSSION
Our study shows that the LifeFirst program was successful in reducing tobacco and supari use among students attending the corporation schools in Mumbai. It not only led to reduction in tobacco and supari use but also protected students in the intervention group against new uptake of tobacco. Additionally, it also helped improve knowledge score as well as refusal skills among students. The knowledge of health harms was considerably high to begin with in both the groups. Despite this, students were using some tobacco or supari products. Thus, this implies that only high knowledge of health harms is not sufficient as a solution, and this has to be combined with cessation support to result in behavior change.
We believe our study adds significantly to limited evidence available in India for school-based tobacco control interventions. While there are many school-based tobacco control programs in India, including school-based components of the National Tobacco Control Program, there are only a few focused on behavior modification interventions. And among school-based programs, there is a dearth of published evaluative research on impact of these programs. While not strictly comparable with the LifeFirst program, an evaluation of a multicomponent school-based tobacco control intervention had shown reduction in tobacco use (especially, cigarette and bidi smoking) as well as intention to take up tobacco use in future among 6th and 8th grade students in Delhi and Chennai cities over the twoyear intervention period19. Beyond this, we were unable to find other published evidence on school-based interventions in India comparable to the LifeFirst program. This implies not only the potential of school-based interventions for reducing tobacco and supari use among youth, but also the need to do evaluative research around ongoing school-based interventions in India.
The LifeFirst program specifically engaged with students in corporation (local government) schools that generally come from lower socioeconomic class. Youth in general and those coming from lower socioeconomic classes in particular are more vulnerable to tobacco use32,33. Furthermore, the program’s emphasis on offering cessation support for tobacco as well as supari use seems to be responding to a large gap in such support services for students in India. While the number of students using tobacco was limited in the LifeFirst cohort, there was a huge diversity of products that were used by students, including surprise reports of use of e-cigarettes (referred to by students as ‘pen-hukka’) by many students. It highlights how the tobacco industry targets young people and the relevance of the regulatory measures by governments prohibiting e-cigarettes (electronic nicotine delivery devices) in India to protect youth15. The program also shows the importance of addressing a high prevalence of supari use among youth. Our findings show that during the course of the study many students quit supari use but began using tobacco. This further strengthens the belief that detailed studies are warranted to establish if supari is a gateway to tobacco use among adolescents9,10,34.
Strengths and limitations
While a robust experimental design with high response rate and the use of difference-in-difference analysis reflects the strength of our study, there are certain limitations too. We ended up with a limited number of tobacco users (about 17%) in our sample population, with the majority being supari users. While we still find statistically significant change in our outcome variable, the small number limits the possibility for further analysis segregated by gender and age – something we would have liked to do. We did not use biomarkers owing to the study context and study population and relied on self-report to assess tobacco and supari use status by students. Selfreported responses may be subject to social desirability bias, but as we use the same measures (assessed through the same questionnaires) over time and across groups, we believe that a comparative analysis would still provide a reliable measure of change over time. Self-reported current tobacco use among adolescents has been found to be a valid and stable indicator that can be suitable for public health research35. Finally, our sampling (and the explicit LifeFirst program strategy) presents a self-selection bias where tobacco and supari users willingly register for the program. Hence, our encouraging results should be read in the context of student users who are willing to quit tobacco and/or supari and cannot be generalized to all student users.
Implications
Our work with school-based interventions implemented in schools across Mumbai suggests that structured, long-term tobacco and supari cessation interventions within school settings could help young people quit their tobacco use and prevent initiation of tobacco use.
We argue that there is a need for more such interventions as part of a multi-strategy approach to reducing tobacco and supari use and a need for systematic evaluation of these interventions.
CONCLUSIONS
The LifeFirst school-based tobacco and supari cessation program appears to be effective in helping students using tobacco and supari to stop their habit. For future initiatives, we believe adding a component of cessation (using content and activities suited for adolescents) to interventions aimed at improving knowledge of students will augment the impact of such programs.