INTRODUCTION
Countries have taken heterogeneous approaches to account for immunization in their national health budgets and expenditures; however, evidence for best immunization finance practices is limited1. There is increasing evidence showing the diversion of resources alongside budget reallocation to counter existing universal healthcare services and systems strained by COVID-19, such as routine immunization programs2,3. As the world emerges from the disruptions caused by the pandemic, there is an urgent need to re-focus the attention on the control and prevention of other vaccine-preventable diseases (such as measles or pertussis) to recover lost ground and to equip immunization programs with the capacity to withstand unforeseen shocks.
The Immunization Agenda 2030: A Global Strategy to Leave No One Behind (IA2030) framework, proposed by the World Health Organization (WHO), delineates a series of objectives to guide countries to build upon the progress that has been made through the Global Vaccine Action Plan 2011–20204. The overall aim of the IA2030 is for all countries to have a reliable supply of appropriate and affordable vaccines of assured quality and sustainable financing for inclusive immunization programs5. The objectives stated in the framework are to build and maintain healthy global markets across all vaccine antigens, to ensure sufficient financial resources for immunization programs in all countries, and to increase immunization expenditure from domestic resources in aid-dependent countries and when transitioning away from aid, and secure government funding to achieve and sustain high coverage for all vaccines4.
This narrative review aimed to review the literature regarding different strategies for sustainable financing of immunization programs and how they fit within the IA2030, and to propose strategic interventions to increase the sustainability and resilience of existing vaccination infrastructure in the post-COVID era4. We conducted searches through the PubMed database from 16–18 March 2023. A detailed search strategy (Supplementary file Table 1) was developed using MeSH terms and free-text keywords (i.e. investment, financing, immunization/vaccination programs, resilience, sustainability). Reference lists of publications that satisfied the eligibility criteria were hand-searched for additional articles not identified by the search strategy and yielded three additional publications. The search was supplemented with Google’s advanced search to also include relevant grey literature.
The included articles were restricted to published literature in peer-reviewed journals written in English and published within the past three years, from January 2020 to February 2023. The timeline was set to capture relevant publications and reports generated after WHO’s declaration of the COVID-19 outbreak as a public health emergency of international concern. Articles were selected based on relevance to identifying strategies that contribute to sustainable financing of immunization programs. Selected articles had an international or regional focus. Publications that specifically focused on the vaccination landscape of an individual country or on a single vaccine, were excluded. Editorials, viewpoints, commentaries, and modelling studies were also excluded. We did not include any publications that considered non-human or hypothetical vaccines.
Publications, from the PubMed database search, were screened based on title and abstract. Two independent reviewers read selected articles in full and considered them for inclusion based on pre-established eligibility criteria. The screening and selection process is presented in Figure 1. Disagreements over including relevant publications were resolved through discussion with a third independent reviewer. For data extraction and analysis, thematic analysis was conducted from March to April 2023 using Excel based on the conceptual framework proposed in The Immunization Agenda 2030 (IA2030) Framework for Action, in which supply and sustainability are key strategic priorities5.
Figure 1
PRISMA flow chart outlining screening and selection process of English-language peer-reviewed articles, focusing on sustainable financing strategies for immunization programs not related to COVID-19 at an international or regional level, included in literature review
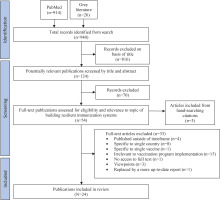
The PubMed search identified 914 potential articles, and the grey literature search resulted in 26 potential articles. Of the total records (n=940) identified from the search, 816 were excluded based on the title. A total of 124 publications were screened by title and abstract; of these, 54 were assessed for eligibility and relevance to the topic. Full-text articles (n=33) were excluded based on being published outside of the timeframe (n=4), specific to a single country (n=8), specific to a single vaccine (n=1), irrelevant to vaccination program implementation (n=15), no access to the full text (n=1), being viewpoints (n=3), or replaced by a more up-to-date report (n=1). Thus, of the 54 articles screened for eligibility and relevance to the topic, 24 articles met the criteria and were included in the final sample. Included from the grey literature were eight reports – yielded from the supplementary grey literature search and were reports on post-pandemic investments in preventative health and immunization services published by reputable public health and academic institutions, such as the WHO, the World Bank, the Coalition for Epidemic Preparedness Innovations (CEPI), International Monetary Fund (IMF), and the Organization for Economic Co-operation and Development (OECD), six reviews – of which one was a meeting summary, one a proceeding from a workshop, two systematic reviews, two consensus study reports, one scoping review, one narrative literature review, two mixed-methods – of which one was survey and interventions/focus group discussions, and the other a literature review followed by interviews, and one working paper. Characteristics of included studies can be found in the Supplementary file Table 2.
STRATEGIES FOR SUSTAINABLE FINANCING OF IMMUNIZATION PROGRAMS
Strategies analyzed were organized into four key areas, following the IA202304: 1) sufficient, predictable resources; 2) effective public financial management; 3) collaborative partnerships; and 4) sustainable transition from external donor support. Using this pre-existing framework, the evidence in the literature about financing strategies being used today and recommendations for financing future vaccination programs are presented in Table 1.
Table 1
Summary of recommendations for sustainable financing for immunization as adapted from the Immunization Agenda 2030 Framework
Regarding ensuring sufficient, predictable resources, two subthemes, or strategic interventions, were identified. The first was increasing domestic government public funding1,6-8, where key findings included designing health financing strategies that prioritized primary or universal healthcare initiatives1,6. The second was the need to diversify sources of financing1,6,9-14. Three strategies were identified, namely: realizing the potential of public-private partnerships (PPPs) to align priorities across different sectors1,6; the creation of incentives to improve access and affordability, such as initiatives like the Market Information for Access to Vaccines (MI4A)11 or pooled resources for pull/push mechanisms12,13; and increasing coverage and supporting vaccine research and development (R&D)12-14.
Increasing domestic government public funding
Increasing domestic government public funding is essential for prioritizing immunization within primary healthcare1,6. Governments are encouraged to allocate more resources by effectively communicating the socio-economic value of increasing their financial support for developing and funding vaccination programs7,8. Robust economic evaluations, such as Financial Value of Vaccine Assessments (FVVA), offer a multi-perspective approach that considers varying contexts, such as demographics, healthcare capacity, and competing priorities, helping governments make informed decisions about resource allocation7,8.
Diversify sources of financing
Broadening the funding base allows countries to better secure the necessary resources for their immunization programs. Therefore, collaborating with a variety of alternative stakeholders, such as investment banks, development banks, service clubs, and private investors, enhances the availability and sustainability of vaccines9,10. PPPs are crucial in improving vaccine pricing transparency, enabling better-informed decision-making in vaccine procurement and introduction strategies1,6. Initiatives like the MI4A provide valuable market information that improves decision-making and reduces vaccine cost11. Creating incentives through pooled resources for pull and push mechanisms can stimulate vaccine research and development (R&D)12,13. Supporting R&D efforts, such as developing new vaccine platform technologies, adjuvants, and alternative administration routes, permits countries to foster innovation and strengthen their immunization programs12-14.
For effective public financial management, the strategic interventions identified in literature were: improving vaccine demand forecasting, budgeting, and procurement8,10-12,15-20, where accurate market assessments11,12,15 and ensuring reliable cost and expenditure data by mapping budget processes11,18-20 were highlighted as critical findings; establishing credible regulatory bodies10-12,16,17,19-21, to expedite approval processes11,12 and evaluate program targets10,19; and alternative financing models and funding strategies1-3,6,7,10-12,14,17,19,21,23,25,26, such as Advanced Market Commitments (AMC)1,11,12,17, investing in R&D1,12,21,25,26 and incentives for procurement1,12,17.
Improving vaccine demand forecasting, budgeting, and procurement
Due to the heterogeneity in population needs and healthcare budgets, there is limited understanding of how countries manage immunization within their health budgets. There is no universal solution. Regardless, the literature argues that effective public financial management is crucial for ensuring the efficient allocation and utilization of resources in the context of vaccine procurement and distribution15. By strengthening institutional capacity for planning, budgeting, disbursement of funds, and accountability, countries can enhance their ability to handle vaccination within their health budgets8,10,11,16-19. Accurate market assessments are crucial to improve vaccine demand forecasting, budgeting, and procurement11. Conducting comprehensive assessments driven by country needs that involve multiple stakeholders allows for a deep understanding of specific vaccine requirements, enabling informed decision-making11,12,15. This, in turn, enables informed decision-making, allowing for the development of targeted strategies that meet vaccine demand, all while ensuring the availability of affordable and quality vaccines13,15. Reliable cost and expenditure data underpin evidence-based budgeting, fostering efficiency and transparency11,18. Budget execution problems can be managed by mapping budgeting processes; countries can identify bottlenecks, streamline processes, and enhance financial performance19,20. Dynamic vaccine budgets with a horizon-scanning approach facilitate effective planning and resource allocation, allowing swift responses to changes in vaccine demands16,19. Special or extra-budgetary funds, repurposed existing funds, pooled revenue, supplementary development assistance, and contingency or reserve funds provide this necessary flexibility17,19.
Establishing credible bodies
Trust and efficacy in vaccination programs rely on transparent, standardized regulatory frameworks11,12,16,20,21. Leveraging credible regulatory bodies, such as the National Immunization Technical Advisory Group (NITAG) and the National Regulatory Authority (NRA), enables countries to make informed decisions to optimize resource allocation and maximize the impact of vaccination efforts11,12,17. Collaboration between these plays a crucial role in fast-tracking vaccine introduction, providing evidence-based advice on new vaccine introductions, product choices, and delivery strategies11,12. Removing barriers to harmonization and standardizing registration processes allows countries to expedite processes, allowing vaccines to be available when most needed11,12.
Credible regulatory bodies play a crucial role in ensuring vaccine safety, efficacy, and acceptance; they allow countries to understand the factors affecting vaccine acceptance and tailor immunization programs to meet population needs7,9,18,22,23. Effective communication strategies, such as community engagement and culturally appropriate information, are crucial to address vaccine hesitancy and enhance uptake and coverage7,8,11,24.
Alternative financing models and funding strategies
An example of AMC negotiations that highlight this is the case of pneumococcal conjugate vaccines (PCVs) in LMICs. PCVs are pivotal in enhancing vaccine accessibility and reducing barriers to vaccine uptake, but their high cost and limited availability limit their use in resource-constrained settings23. To overcome these challenges, Gavi and the WHO collaborated to launch the AMC for PCVs in 200912. Guaranteeing a volume of PCV doses at reduced doses provided manufacturers financial security, enabling them to scale up production and establish distribution networks23. Gavi leveraged various resources from public and private donors, negotiating favorable prices and securing long-term supply commitments12.
Investing in innovations is crucial1,6,10,19. By investing in R&D, governments and organizations can drive advancements in vaccine administration and delivery, new vaccine technologies, and interventions against different pathogens1,14,21,25,26. Push/pull incentives and non-dilutive funding can be utilized to de-risk vaccine R&D1,7. Pull incentives have been demonstrated to be effective for late-stage vaccine development7. These incentives provide financial support and create a more predictable market, encouraging private investment.
Incentives for procurement are essential to strengthen the bargaining power of public procurers and ensure a steady supply of vaccines1,12,17. Strategies include multiple winners’ tenders, market risk sharing, direct loans to small manufacturers, subsidies from private donors, and milestone payments for achieving specific results12,17. Including vaccine supplies for low-income countries (LICs) as components of grants and contracts with vaccine developers, enables pre-purchasing and reduces the risk of shortages. Providing up-front, at-risk manufacturing to global procurers, such as the Global Alliance for Vaccines and Immunization (Gavi) and the Global Fund, allows for large-volume pre-purchasing, ensuring the timely availability of vaccines.
Pooled procurement mechanisms (PPM) have emerged to ensure equitable access to COVID-19 vaccines, although with varying success1-3,6,7,10,11,19. By leveraging collective purchasing lower, negotiating favorable pricing arrangements with vaccine manufacturers becomes a reality – enhancing procurement efficiency1,2. However, in the context of COVID-19, challenges related to supply chain vulnerabilities and uneven recovery efforts across different regions have been observed6,7. These highlight the complexity of coordinating various stakeholders alongside the need to strengthen procurement strategies, especially in lowresource settings11.
For collaborative partnerships, strategic interventions include: equitable access1,6,7,9,15,17,19,22,23,27 – achieved through adherence to donor commitments and pledging systems1,6,7,9,15, building equitable access clauses into future AMC negotiations22,23, ensuring a degree of governmental ownership6,27, and diversifying the Developing Countries Vaccine Manufacturers Network (DCVMN)17,27; strong collaborative action1,6,13,16,19,28 – enabled by streamlining partnerships1,16,28, engaging the private sector in immunization efforts1,16,19, mobilizing adequate funding1,16, and conducting landscape analyses1,16,19 ; and sharing of know-how and technology transfer7,8,15-17,20,21,28, enabled by the creation of a manufacturers’ portfolio15 and a common framework for industry-industry or government-industry partnerships7, ensuring adequate disease surveillance15,20,28, and reassessing the intellectual property landscape15.
Equitable access
Equitable access can be achieved through adherence to donor commitments and pledging systems, enabling long-term immunization planning1,6,7,9,15. Cross-country sharing of best funding practices is another strategy1,9. Different countries may have unique approaches for funding immunization programs effectively. Sharing these allows governments and stakeholders to gain insights into successful funding strategies; it enables the identification of funding strategies that can be adapted and applied to different contexts.
Another strategy is the building of equitable access clauses into future AMC negotiations22,23. These are mechanisms that incentivize the development and availability of vaccines for diseases disproportionately affecting LMICs. These clauses address fair pricing, IP licensing, expanded registration, and clinical trials. As suggested by the IMF, the creation of an Advanced Commitment Facility with zero-day financing could empower LMICs by enabling their entrance into advance purchase agreements for vaccines22. By providing early-day financing to support vaccine procurement, the availability of vaccines to LMICs would be ensured, and access would be expanded, all while leveraging PPPs to address financial and supply challenges.
Governments should maintain a degree of ownership in developing or manufacturing vaccines when providing public funding or pull incentives6,27. This ownership provides governments with leverage to influence affordable pricing, encourage the licensing of intellectual property, and mandate expanded registration and supply, ensuring that vaccines meet the population’s needs.
Strong collaborative action
Collective action to streamline and coordinate partnerships across national, regional, and global levels is crucial to fostering sustainable financing for immunization programs1,16,28. Working together enables the pooling of resources, expertise, and funding – ultimately allowing the sharing of best practices, lessons learned, and innovative strategies to improve immunization programs.
Moreover, collective action is essential to reach the call for investment of US$21 billion for diagnostics, therapeutics, and vaccines1,16. By mobilizing the necessary funding, governments and international organizations can ensure that immunization programs are adequately supported and maintain a reliable supply of vaccines.
To achieve sustainable financing, governments must identify creative ways to engage the private sector in immunization efforts1,16,19. Private sector involvement can bring additional resources, expertise, and innovation to strengthen vaccine R&D, procurement, manufacturing, and distribution6,13. By aligning efforts and maximizing the impact of private sector involvement, governments can leverage the expertise and resources of private companies to enhance immunization programs. To do so, landscape analyses can be conducted1,16,19. These delineate the role of the private sector and identify areas of maximum effect through assessing the landscape of vaccine development, production, and distribution. They provide valuable insights for decision-making and enable governments to harness the private sector’s potential in advancing immunization goals.
Sharing of know-how and technology transfer
By sharing know-how, infrastructure and manufacturing capacity can be enhanced on a large scale – leading to an increased vaccine supply and access7,15,16. This allows for equitable access and fairer allocation of vaccines. Moreover, building multiple regional manufacturing hubs with a more balanced geographical distribution can circumvent supply chain failures or export restrictions15. These hubs can focus on producing regionally necessary vaccines and transfer the technology to existing or new manufacturers.
Another component of sharing know-how is adequate disease surveillance15,20,28. Establishing surveillance systems at different levels enables countries to monitor disease trends, detect outbreaks early, and inform targeted immunization strategies. This includes investing in surveillance infrastructure and promoting data sharing and collaboration between different stakeholders.
Creating a manufacturers’ portfolio will guarantee supply safety and foster long-term competition between manufacturers15. This is done by ensuring multi-manufacturer tendering and promoting cooperation between members. In addition, partnerships between the original vaccine manufacturer and companies in LMICs can facilitate local production in distant regions, ultimately permitting adequate population coverage7. The self-sufficiency of LMICs can be enhanced through the DCVMN, which is key to achieving sustainable financing for immunizations15,17.
A framework for industry-industry or government-industry partnerships is crucial to coordinate partnerships and organize vaccine R&D funding portfolios7. This includes identifying promising technologies and sharing production and yield data. Regional partnerships can contribute by harmonizing regulatory frameworks, standards, and licensing requirements for importing medical devices and medicines8,15. Streamlining these processes allows for an enhanced collaboration with subsequent efficient and effective vaccine development and distribution.
Finally, the intellectual property landscape needs to be reassessed to address barriers in R&D, procurement, and manufacturing15. Initiatives such as voluntary licensing, patient pools, technology access pools like the COVID-19 Technology Access Pool, and tech transfer hubs, like the WHO mRNA Tech Transfer Hub, facilitate technology sharing and access. Identifying support processes that foment the management of patents and freedom to operate in the development of vaccine products, also enables smooth progress in vaccine research and manufacturing21.
The primary strategic intervention identified for building sustainable vaccination programs includes a sustainable transition away from donor assistance1,7,15-17,19. Key findings include early engagement with country-level stakeholders and alignment with the country’s long-term plans and priorities1,19, flexible support to account for unique circumstances17,19, and integration with other country-level health priorities19.
Sustainable transition away from donor assistance
Timelines for transitions out of donor support should align with a country’s national medium and long-term plans and priorities1,19. Establishing good governance practices that hold stakeholders accountable and ensure transparency in funding and governance is essential. To do so, the engagement of all relevant stakeholders from the early stages of the planning process, alongside fostering local ownership, is required1,19.
External donor assistance should complement in-country efforts to strengthen national immunization programs within existing PHC infrastructure, enabling countries to leverage their domestic funding15,16. This assistance can be targeted support, technical assistance, and reducing financial barriers. Flexibility is another crucial aspect in transitioning from external donor support17,19. External donors should provide flexible support whenever possible, such as adjusting transition timelines to accommodate unique circumstances or reallocating funds to meet changing needs and priorities. Countries may also need to explore alternative sources of financing, such as accessing funds from multilateral development banks and seeking support from other donors in the transition period.
Finally, the integration with other country-level health priorities is crucial to enhance cross-programmatic efficiency19. Resources can be optimized through the aligning and harmonizing of investments. This integration promotes a comprehensive healthcare approach that maximizes the impact of investments and resources.
Key implications for government and planning
Our narrative review aimed to review the literature regarding different strategies for sustainable financing of immunization programs and how they fit within the IA2030. Based on the above results, the key implications of this study for government and planning are outlined below.
Ensuring sufficient predictable resources
Integrating strategies such as bottom-up preparedness, local ownership, and effective communication encourages increased domestic government funding. This synergistic approach, supported by robust economic evaluations like the Financial Value of Vaccine Assessments (FVVA), ensures that allocated resources are utilized effectively and efficiently, emphasizing community involvement for sustained impact.
Effective public financial management
Optimal resource allocation8,10,11,16-19
Strengthening institutional capacity and financial management frameworks enhances efficiency in resource allocation for vaccine procurement and distribution. Accurate market assessments, driven by country needs and stakeholder involvement, improve vaccine demand forecasting, budgeting, and procurement. Integrating disease surveillance and information systems further optimizes resource allocation, enhancing responsiveness to disease threats. Dynamic vaccine budgets with a horizon-scanning approach ensure efficient planning and allocation, while establishing credible regulatory bodies fosters trust and streamlines resource allocation for vaccination efforts.
Transparent processes for timely access11,12
Transparent and standardized regulatory frameworks, along with expedited approval processes, contribute to the timely access to vaccines. Standardized registration processes facilitate rapid vaccine availability when needed.
Diver funding for sustainability1,11,12,17
Integrating alternative financing models like Advanced Market Commitments (AMCs) promotes equity by reducing barriers for low- and middle-income countries. Investing in innovations, supported by push/pull incentives, fosters research and development, ensuring sustained funding and advancements in vaccine technologies.
Diversification for risk reduction12,17
AMCs and diversified funding sources enhance efficiency in resource allocation, reducing the risks of shortages and ensuring a steady supply of vaccines. Collective procurement mechanisms negotiate favorable pricing arrangements, contributing to the efficiency of resource allocation.
Collaborative partnerships
Donor commitments and equitable access clauses1,6,7,9,15,22,23
Achieving equitable access involves donor commitments, cross-country sharing of funding practices, and integrating equitable access clauses into future AMC negotiations. Governments maintaining ownership and collaboration in vaccine development foster fair pricing and expanded access for populations.
Pooling resources for sustainable financing1,16,28
Collective action across national, regional, and global levels fosters sustainable financing, pooling resources to share best practices and innovative strategies for improving immunization programs. Mobilizing funds ensures adequate support and a reliable vaccine supply.
Regional collaboration for access7,15,16
Sharing know-how enhances manufacturing capacity, promoting a more balanced geographical distribution of regional manufacturing hubs. By focusing on regionally necessary vaccines and technology transfer, LMICs achieve self-sufficiency, ensuring equitable access and fairer allocation of vaccines.
Sustainable transition and future preparedness
Transitioning with governance and integration1,15-17,19
Timelines for transitioning away from donor assistance align with national plans, emphasizing good governance practices, stakeholder engagement, and local ownership. Integrating immunization efforts with other health priorities ensures cross-programmatic efficiency, maximizing the impact of investments and resources for long-term sustainability.
Implementing these strategies enables countries to enhance the resilience and sustainability of their immunization programs – ultimately ensuring equitable access to vaccines and improved health outcomes.
Strengths and limitations
The strengths of this review include the thematic analysis of strategies, whereby – within each key area – the review conducted a detailed thematic analysis of strategies, providing nuanced insights into specific interventions. The review also incorporated evidence from various sources to formulate evidence-based guidance for policymakers and governments. Moreover, the review identifies strategies and emphasizes their contextual relevance. For instance, recognizing the heterogeneity in population needs and healthcare budgets underscores the importance of tailoring strategies to specific country contexts.
This review has limitations. First, the database search was limited to PubMed, a comprehensive database. Moreover, the peer-reviewed literature was supplemented by a relevant grey literature search and study identification. Secondly, given the narrative nature of the review (not systematic) – no quantitative analysis was performed, and we did not assess for publication bias in the identified studies. Finally, the current review only included articles written in English as per the language filter applied during the search. While further research with a systematic approach would be useful for the formulation of evidence-based policies, this narrative review provides a brief overview of broad areas that could be taken into account when assessing different strategies for the sustainable financing of immunization.
CONCLUSION
This review advocates for a synergistic approach to bolster government planning for immunization programs. Emphasizing bottom-up preparedness, local ownership, and effective communication encourages increased domestic government funding, supported by robust economic evaluations like the FVVA. Effective public financial management involves strengthening institutional capacity, accurate market assessments, and integrating disease surveillance systems. Transparent processes, expedited approval mechanisms, and diversified funding sources, including AMCs, contribute to timely vaccine access and sustainable funding. Collaborative partnerships, marked by donor commitments and equitable access clauses, are pivotal in achieving global immunization goals. Lastly, the review underscores the importance of transitioning away from donor assistance through good governance practices, stakeholder engagement, and integration with broader health priorities, ensuring long-term sustainability and improved health outcomes globally.


