INTRODUCTION
Early initiation of and adherence to antiretroviral therapy (ART) can improve health outcomes among people living with HIV (PLHIV) and prevent onward HIV transmission1-4. The global 90-90-90 targets set out by the Joint United Nations Programme on HIV/AIDS (UNAIDS) are to diagnose 90% of all PLHIV, provide ART for 90% of those diagnosed, and achieve viral suppression for 90% of those on ART5. Ensuring ART is taken as prescribed is vital to achieving the third target; yet many PLHIV face numerous treatment challenges that can sabotage adherence6-11.
Because daily oral ART is currently the sole option for HIV treatment, many problems arise because of either the frequency (i.e. daily) or route of administration (i.e. oral)12. Daily intake can be a source of concern regarding unintentional disclosure of HIV status or serve as an unwanted reminder of HIV13,14. Drug-drug and drug-food interactions, as well as pill aversion, can also complicate daily oral ART12,15-17.
With injections every two months, long-acting cabotegravir and rilpivirine (CAB LA + RPV LA) is an innovative HIV treatment regimen designed to provide PLHIV with an alternative that is as effective as daily oral ART18,19. In a 25-country survey of PLHIV, the long-acting attribute was highly favored as a key ‘improvement to HIV medicines’, second only to improvements in the long-term safety profile of medicines20. In that same study, 54.7% of participants indicated preference for long-acting regimens, especially those with confidentiality concerns, suboptimal adherence, and gastrointestinal ART side effects20. In qualitative interviews of patients being treated with CAB LA + RPV LA in the phase IIb clinical trial LATTE-2, patients described the regimen as being more ‘discreet’ than pills, with less opportunity for stigma, discrimination or undesired disclosure21.
Given the anticipated change in treatment paradigm because of the advent of long-acting regimens18,19, it is important to quantify problems related to daily oral dosing to determine who may benefit most from long-acting regimens. While some problems have been previously identified12-17,20,21, the populations in some of those studies were either PLHIV alone22-24 or healthcare providers (HCPs) alone25,26. This may not provide a complete picture of problems associated with treatment as attitudes and perceived priorities of patients versus providers may not always converge perfectly. Patients may not fully communicate with their HCPs treatment concerns, including emotional or personal issues, which may be contributing to poor adherence27; HCP communication may also be limited, despite its key role28,29.
As a prelude to this current study and building upon the work in the literature, we undertook formative research among HIV patients and providers to review, refine and define the categories of problems in relation to daily oral ART dosing, with the aim of better capturing and quantifying them in the present study. From the advisory boards, qualitative interviews, and pilot surveys conducted, four main categories of problems emerged: 1) medical conditions interfering with daily oral administration, 2) suboptimal adherence, 3) confidentiality concerns, and 4) emotional wellbeing related to daily tablet requirements. We explored these objectives from two complementary perspectives – that of PLHIV and HIV physicians – as some categories of unmet needs are better informed by patients (confidentiality, emotional wellbeing) whereas HCPs are better placed to report proportion of patients with specific medical conditions.
METHODS
Study population
This study was sponsored by ViiV Healthcare and fielded by Ipsos Healthcare. Web-based surveys of 120 physicians and 698 PLHIV were conducted from June to August 2019 in Germany, Italy, UK, and France. These countries were selected because of their high HIV burden in the European Economic Area30, together accounting for over half of all new HIV diagnoses during 201611. The logistical challenges of recruiting a statistically robust sample size in smaller European countries made it challenging to perform this study in additional countries. Because of the non-probabilitybased sampling approach used in this study, the target population and the analyzed population are synonymous. Yet, inferences may be transferrable to PLHIV populations in other geographical locations that have similar challenges and shared experiences.
Inclusion criteria
PLHIV inclusion criteria were: 1) able to confirm selfreported HIV status with either a photo of their HIV medication or their prescription; 2) resident of surveyed country and able to read, speak, and understand the official language; and 3) willing and able to provide electronic informed consent.
HCP inclusion criteria were: 1) board certified or eligible physician directly involved in the treatment of adult PLHIV; 2) practiced internal medicine, HIV medicine, or infectious disease specialty for ≥5 years; 3) personally managed ≥50 HIV patients; 4) consulted ≥15 HIV patients per week; 5) resident of surveyed country and able to read, speak, and understand the official language; and 6) willing and able to provide electronic informed consent.
Recruitment
Approximately 60–70% of all PLHIV in this study came from existing panels; these were pre-screened individuals with a confirmed diagnosis of HIV who were ready and willing to participate in research studies. Use of these existing panels allowed for efficient recruitment of participants in the different countries, based on inclusion criteria. The remaining 30–40% of participants were actively recruited using different sources affiliated with PLHIV, including nongovernmental organizations, patient support groups; other national, regional, and local charities/support groups; online support groups/communities, social media (Facebook), and direct patient referral (snowball sampling). Among all active recruits, HIV diagnosis was ascertained and confirmed using a photo of PLHIV’s ART medication or prescription with their name on it. All personal identifiable information was discarded after confirming HIV status. To recruit PLHIV, Ipsos Healthcare partnered with two companies, Liberating Research, and Opinion Health, which specialize in recruiting, sourcing, and operating on-going panels/ communities of people who live with different conditions. Liberating Research recruited participants from all four countries, contributing 84.8% of all study participants combined (66.7% in France, 80.2% in Germany, 82.2% in Italy, and 100.0% in the UK). The remainder came from Opinion Health. Ipsos Healthcare monitored PLHIV recruitment on a weekly basis to ensure that the recruited sample’s composition aligned with the national HIV population on key characteristics (age, gender, sexual orientation, and country of origin)11.
HCPs were recruited as a purposive sample, a form of non-probability sampling. Panels of previously profiled HCPs with larger caseloads of PLHIV were used. The rationale for oversampling HCPs with many HIV patients was to ensure that we could generate robust estimates for certain outcomes among their managed patients that were assumed to be rare outcomes (e.g. certain medical conditions interfering with oral administration). Secondarily, oversampling HCPs with many HIV patients ensured that providers’ perspectives being captured were representative of a diverse array of patients. The participating HCP panelists were sourced from three separate companies that operate on-going panels of providers – Sema, Ipsos (UK/Italy), and Medefield. Sema contributed 8 of 30 HCP participants from France, and 1 of 30 participants from the UK. Ipsos contributed 22 of 30 participants from France, all 30 participants from Italy, and 29 of 30 participants from the UK. Medefield contributed all 30 participants from Germany.
Questionnaire development
The survey themes were guided by formative research and shaped with input from an advisory panel comprising PLHIV, HIV physicians, and patient advocates. The draft questionnaires developed with the help of this advisory panel, were reviewed by local teams in the respective countries to ensure they were culturally and contextually appropriate (e.g. therapies listed in the questionnaire were consistent with those locally available), and translated into the official language (from English to French, German, and Italian) by medically trained translators. Additional trained translators proofread the original translations for accuracy. The questionnaires were then piloted in each of the four countries amongst a small sample of PLHIV and HCPs separately, to ensure comprehension, clarity, and online usability. During this process, respondents self-completed the online questionnaire while sharing their device screen with (and speaking to) a moderator via WebEx. The online questionnaire was optimized for different types of electronic devices (e.g. mobile, tablet and laptop/desktop) and different operating systems (e.g. IOS, Android).
Survey launch
Selected participants were sent an electronic mail invitation to participate. Responses were anonymous and could not be linked back to the participant. All patients were required to electronically provide informed consent for participation in this study and meet the inclusion criteria before proceeding to the screener and main survey. At the time of consenting, each participant was assigned a unique identification number (ID). Only the unique ID was recorded and linked to collected data. Upon completion of the questionnaire, respondents were remunerated for their participation (approximately £20 GBP). The weighted average overall response rate was 64.3% for PLHIV. This study was deemed exempt-research by the Pearl Institutional Review Board (Study number 19-IPSO-125).
Measures
The survey collected information on several clinical and demographic variables important to interpreting and placing into context the rest of the survey results. Viral suppression was assessed with the question: ‘How does your doctor describe your current viral load?’. Those answering ‘Undetectable/virologically-suppressed’ (i.e. viral load under 50 copies per mL) were classified as ‘virally suppressed’.
Current ART regimen was based on the core agent reported (non-mutually exclusive categories), as an integrase strand transfer inhibitor (INSTI), a boosted protease inhibitor (PI), or a non-nucleoside reverse transcriptase inhibitor (NNRTI). A past history of resistance to ART was said to be present if this was reported as the reason for the respondent having stopped ART, switched ART, or failed to achieve viral suppression; or if the respondent was currently on Fuzeon (enfuvirtide), whose main indication is for treatment-experienced patients with evidence of HIV-1 replication despite ongoing ART31. Detailed measurements of the four broad categories of problems related to daily oral dosing are presented below and in Supplemental Table 1.
Table 1
Healthcare providers (HCPs) who participated in the study – demographic and practice characteristics overall and by country
Medical conditions interfering with daily oral administration
In the HCP survey, the following four groups of medical conditions were measured: a) malabsorption; b) gastrointestinal issues interfering with oral administration; c) difficulty in swallowing (e.g. phobia or pill aversion, esophagitis, mechanical obstruction, excluding central nervous system disorders); and d) neurocognitive conditions. For each of these conditions, HCPs quantified the estimated prevalence among HIV patients: ‘Based on your experience and knowledge overall’. Individual conditions within the PLHIV survey were grouped into those same four broad categories as the HCP survey (Supplemental Table 1). PLHIV were also asked if they experienced problems while taking ART with other medications, food, or recreational drugs.
Daily oral ART and challenges with adherence
Adherence was considered ‘not only in terms of missed doses but also taking the pills at the right time and under the right conditions without overdosing’. In the HCP survey, physicians estimated what percentage of their patients had suboptimal adherence. In the PLHIV survey, self-reported frequency of failing to take HIV medication exactly as prescribed, as ‘Sometimes’, ‘Often’, or ‘Very often’, was classified as suboptimal adherence.
Confidentiality concerns associated with daily oral ART
Similar questions were asked in the HCP and PLHIV surveys regarding patients’ attitudes and behaviors towards sharing of their HIV status with others, hiding of HIV medication to prevent unwanted disclosure, and perceived stigma. HCPs who reported the perceived frequency of privacy concerns among HIV patients as ‘Often’ were classified as perceiving it as prevalent. Among PLHIV, responses of either ‘Yes’, or reported frequency of ‘Sometimes’/‘Often’/‘Very Often’ were classified as positive responses.
Emotional wellbeing related to daily tablet requirements
The HCP and PLHIV questionnaires assessed treatmentrelated challenges, including patients’ emotional wellbeing as well as their concerns about HIV and/or HIV treatment. Covered issues included dosing schedule, perceived short-, intermediate-, and long-term impacts of treatment, concerns about transmitting HIV, and sexual wellbeing.
Statistical analyses
Because these four countries are well-defined geographically and have similar HIV profiles, we conducted pooled analyses of the data30. The surveys were all standardized and fielded at the same time, minimizing measurement and time biases. Statistical comparisons of key characteristics showed no significant differences across countries on several key characteristics including age, marital status, and year of diagnosis. Pooled analyses allowed for increased precision of subgroup estimates.
For the HCP survey, physicians provided their best estimate for the proportion of HIV patients that met a characteristic of interest. For the PLHIV survey, the individual respondent was the unit of analysis, and unless stated otherwise, all analyses were among those currently on ART (n=688). Within-group statistical comparisons were performed using χ2 at p<0.05. All statistical analyses were conducted using R Version 3.6.3.
RESULTS
Half of the participating HCPs worked in general hospitals (including district general hospitals, 50.9%), 14.2% worked in university or medical school-based hospitals, and 9.2% were in private practice. The remainder (39.9%) worked in outpatient clinics, genito-urinary medicine clinics, or other settings. HCPs reported managing a mean of 299 (SD=177) HIV patients, of whom 85.7% were reportedly on ART. Other HCP characteristics and those of their managed patients are given in Table 1.
Of all PLHIV respondents, 98.6% (688/698) were currently on ART. Mean age and duration of HIV among PLHIV on treatment were 40.9 (SD=12.0) and 11.8 (SD = 9.6) years, respectively. Most PLHIV on treatment were: homosexual (60.6%); men (66.4%); with a partner (52.5%); college educated (58.6%); employed (68.3%); living in metropolitan areas (69.5%); virally suppressed (89.4%); diagnosed prior to 2017 (87.2%); and native born (62.4%) (Table 2).
Table 2
Characteristics of adults living with HIV on antiretroviral therapy in four European countries as well as the percentage reporting various medical conditions interfering with daily oral ART dosing, 2019
| Characteristics | Distribution | Malabsorption | Gastrointestinal conditions interfering with oral ART | Dysphagia (difficulty swallowing) | Neurocognitive conditions | ||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | % | p | % | p | % | p | % | p | |
| Total | 688 (100.0) | 8.1 | 18.6 | 17.9 | 35.5 | ||||
| Gender | |||||||||
| Women | 229 (33.3) | 8.3 | χ2(2)=0.19 | 21.8 | χ2(2)=2.75 | 32.3 | χ2(2)=48.4 | 33.2 | χ2(2)=4.31 |
| Men | 457 (66.4) | 8.1 | p=0.911 | 17.1 | p=0.253 | 10.7 | p<0.001 | 36.3 | p=0.116 |
| Other | 2 (0.3) | ¶ | ¶ | ¶ | ¶ | ||||
| Sexual orientation | |||||||||
| Heterosexual | 233 (33.9) | 6.4 | χ2(2)=3.91 | 20.6 | χ2(2)=1.46 | 30.0 | χ2(2)=41.59 | 32.2 | χ2(2)=2.70 |
| Homosexual | 417 (60.6) | 8.4 | p=0.141 | 18.0 | p=0.481 | 10.3 | p<0.001 | 36.5 | p=0.260 |
| Other | 38 (5.5) | 15.8 | 13.2 | 26.3 | 44.7 | ||||
| Age (years) | |||||||||
| <50 | 484 (70.4) | 7.6 | χ2(1)=0.54 | 18.2 | χ2(1)=0.19 | 21.1 | χ2(1)=11.36 | 34.7 | χ2(1)=0.41 |
| ≥50 | 204 (29.7) | 9.3 | p=0.465 | 19.6 | p=0.661 | 10.3 | p=0.001 | 37.3 | p=0.524 |
| Education level | |||||||||
| Postgraduate | 134 (20.0) | 10.4 | χ2(3)=2.72 | 26.1 | χ2(3)=6.86 | 19.4 | χ2(3)=2.97 | 34.3 | χ2(3)=3.08 |
| College | 392 (58.6) | 6.6 | p=0.436 | 18.4 | p=0.076 | 19.1 | p=0.396 | 33.4 | p=0.380 |
| Secondary | 99 (14.8) | 10.1 | 14.1 | 12.1 | 42.4 | ||||
| Other | 44 (6.6) | 9.1 | 13.6 | 15.9 | 38.6 | ||||
| Employment | |||||||||
| Employed | 457 (68.3) | 7.0 | χ2(1)=2.22 | 17.9 | χ2(1)=1.02 | 16.8 | χ2(1)=1.16 | 28.7 | χ2(1)=27.61 |
| Non-employed | 212 (31.7) | 10.4 | p=0.136 | 21.2 | p=0.314 | 20.3 | p=0.281 | 49.5 | p<0.001 |
| Country | |||||||||
| France | 144 (20.9) | 13.9 | χ2(3)=8.58 | 34.7 | χ2(3)=50.29 | 33.3 | χ2(3)=42.79 | 38.9 | χ2(3)=15.04 |
| Germany | 198 (28.8) | 6.1 | p=0.035 | 8.6 | p <0.001 | 6.6 | p<0.001 | 32.8 | p=0.002 |
| Italy | 150 (21.8) | 8.0 | 26.0 | 21.3 | 24.7 | ||||
| UK | 196 (28.5) | 6.1 | 11.2 | 15.3 | 43.9 | ||||
| ART formulation | |||||||||
| Single table | 381 (55.4) | 7.9 | χ2(1)=0.08 | 17.1 | χ2(1)=1.34 | 15.2 | χ2(1)=4.10 | 30.7 | χ2(1)=8.44 |
| Multi-tablet | 307 (44.6) | 8.5 | p=0.777 | 20.5 | p=0.246 | 21.2 | p=0.043 | 41.4 | p=0.004 |
| Self-reported viral status | |||||||||
| Non-suppressed | 73 (10.6) | 12.3 | χ2(1)=1.92 | 45.2 | χ2(1)=38.16 | 60.3 | χ2(1)=99.98 | 38.4 | χ2(1)=0.30 |
| Suppressed | 615 (89.4) | 7.6 | p=0.166 | 15.4 | p<0.001 | 12.8 | p<0.001 | 35.1 | p=0.585 |
| ART side effectsa | |||||||||
| None reported | 344 (50.0) | 5.2 | χ2(2)=10.29 | 11.6 | χ2(2)=32.75 | 2.0 | χ2(2)=208.40 | 27.9 | χ2(2)=17.28 |
| Non-gastrointestinal only | 96 (14.0) | 7.3 | p=0.006 | 14.6 | p<0.001 | 2.1 | p<0.001 | 41.7 | p<0.001 |
| Gastrointestinal | 248 (36.1) | 12.5 | 29.8 | 46.0 | 43.5 | ||||
| HIV diagnosis year | |||||||||
| 2017–19 | 88 (12.8) | 1.1 | χ2(2)=10.07 | 9.1 | χ2(2)=7.05 | 17.0 | χ2(2)=8.37 | 17.0 | χ2(2)=15.46 |
| 2010–16 | 286 (41.6) | 7.0 | p=0.006 | 21.7 | p=0.029 | 22.7 | p=0.015 | 36.7 | p<0.001 |
| Pre-2010 | 314 (45.6) | 11.1 | 18.5 | 13.7 | 39.5 | ||||
| NNRTI-containing ARTb | |||||||||
| No | 450 (65.4) | 7.1 | χ2(1)=1.84 | 17.1 | χ2(1)=1.92 | 14.9 | χ2(1)=7.92 | 33.6 | χ2(1)=2.07 |
| Yes | 238 (34.6) | 10.1 | p=0.175 | 21.4 | p=0.166 | 23.5 | p=0.005 | 39.1 | p=0.150 |
| Entry inhibitorcontaining ARTc | |||||||||
| No | 661 (96.1) | 29.6 | χ2(1)=17.36 | 18.5 | χ2(1)=0.24 | 17.2 | χ2(1)=4.57 | 35.4 | χ2(1)=0.03 |
| Yes | 27 (3.9) | p<0.001 | 22.2 | p=0.622 | 33.3 | p=0.032 | 37.0 | p=0.862 | |
| INSTI-containing ARTd | |||||||||
| No | 300 (43.6) | 7.0 | χ2(1)=0.92 | 16.0 | χ2(1)=2.38 | 18.7 | χ2(1)=0.22 | 34.3 | χ2(1)=0.30 |
| Yes | 388 (56.4) | 9.0 | p=0.336 | 20.6 | p=0.123 | 17.3 | p=0.635 | 36.3 | p=0.585 |
| Protease inhibitorcontaining ARTe | |||||||||
| No | 538 (78.2) | 7.8 | χ2(1)=0.37 | 17.5 | χ2(1)=2.09 | 14.7 | χ2(1)=17.14 | 35.5 | χ2(1)<0.01 |
| Yes | 150 (21.8) | 9.3 | p=0.545 | 22.7 | p=0.148 | 29.3 | p<0.001 | 35.3 | p=0.970 |
| Past ART resistancef | |||||||||
| No | 603 (87.7) | 6.8 | χ2(1)=11.72 | 15.4 | χ2(1)=32.63 | 15.1 | χ2(1)=25.82 | 33.2 | χ2(1)=11.26 |
| Yes | 85 (12.4) | 17.6 | p<0.001 | 41.2 | p<0.001 | 37.6 | p<0.001 | 51.8 | p=0.001 |
ART: antiretroviral therapy (the different classes presented in table are not mutually exclusive); NNRTI: non-nucleoside reverse transcriptase inhibitor. INSTI: integrase strand transfer inhibitor.
a A history of major ART side effects was said to be present if the respondent reported a past adverse effect from HIV medication (e.g. ‘stomach/ gastric problems because of the medication’ or ‘difficulties taking my HIV treatment as I was having too many side effects’), that led to stopping ART, switching ART, or failing to achieve viral suppression from poor adherence, all because of the side effects.
b NNRTI-containing regimens included ‘Atripla® or generics (emtricitabine/efavirenz/tenofovir disoproxil fumarate)’; ‘Delstrigo (doravirine/lamivudine/tenofovir disoproxil fumarate)’; ‘Edurant (rilpivirine)’; ‘Eviplera (emtricitabine/rilpivirine/tenofovir -disoproxil fumarate)’; ‘Viramune or generics (Nevirapin)’; ‘Sustiva or generics(efavirenz)’; ‘Odefsey (emtricitabine/rilpivirine/tenofovir alafenamide)’; or ‘Pifeltro (doravirine)’.
c Entry inhibitor-containing regimens included ‘Celsentri (maraviroc)’; ‘Fuzeon (enfuvirtide)’ or ‘Fostemsavir’.
d INSTI-containing regimens included ‘Genvoya (elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide)’; ‘Tivicay (dolutegravir)’; ‘Triumeq (dolutegravir/abacavir/lamivudine)’; ‘Isentress (raltegravir)’; ‘Juluca (dolutegravir/rilpivirine)’; ‘Stribild (elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate); or ‘Biktarvy (bictegravir/emtricitabine/tenofovir alafenamide)’.
e Protease inhibitor-containing regimens included ‘Kaletra (lopinavir/ritonavir)’; ‘Evotaz (atazanavir/cobicistat)’; ‘Prezista (darunavir)’; ‘Reyataz (atazanavir)’; ‘Rezolsta (darunavir/cobicistat)’; or ‘Symtuza (darunavir/emtricitabine/tenofovir alafenamide)’.
f A history of resistance to ART was said to be present if this was reported as the reason for the respondent having stopped ART, switched ART, or failed to achieve viral suppression; or if the respondent was currently on Fuzeon (enfuvirtide), whose main indication is for treatment-experienced patients with evidence of HIV-1 replication despite ongoing ART.
Medical conditions interfering with daily oral administration
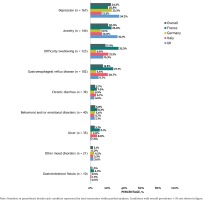
HCP-reported prevalence for different medical conditions among HIV patients was: malabsorption (9.8%); gastrointestinal conditions interfering with oral administration (10.4%); difficulty swallowing (9.7%); and neurocognitive conditions (11.6%) (Figure 1). Categorical distributions of HCP perceived burden of various conditions, depicted in Figure 2, revealed that none to only a small fraction of HCPs perceived any of the assessed problems as being nonexistent (i.e. estimated prevalence of 0%) among HIV patients. Except for malabsorption, PLHIV reported higher rates of these conditions than the corresponding HCP-reported estimates (Figure 1). Prevalence of specific neurocognitive/mental conditions by PLHIV report (nonmutually exclusive) included depression (24.3%; 167/688), anxiety (20.9%; 144/688), and dementia (0.6%; 4/688) (Figure 3). Overall, 18.6% (128/688) of PLHIV reported being diagnosed with a gastrointestinal condition interfering with oral ART administration. Furthermore, 42.5% of PLHIV reported that ‘I must take food at the same time as my HIV treatment’, 23.9% indicated that ‘I cannot take antacids, PPIs or H2 blockers to relieve stomach issues along with my HIV treatment’, and 16.1%, reported ‘I cannot take another drug at the same time as my HIV treatment’ (Table 3). In addition, 19.1% of PLHIV indicated that ‘I need additional monitoring when I take other medications on top of my HIV treatment’ while 15.4% ever ‘had to change at least one drug of my HIV treatment to avoid issues/complications with another drug’ (Table 3). These medical issues were associated with treatment avoidance behavior among PLHIV. For example, 14.7% (101/688) reported they missed their ART dose because they ‘have trouble swallowing pills’, 16.3% (112/688) because of ‘a problem taking pills at specified times (with meals, on empty stomach etc.)’, and 11.2% (77/688) because they ‘were taking another medication’. PLHIV reporting ‘I cannot take another drug at the same time as my HIV treatment’ had almost three-fold higher prevalence than those without this constraint, of missing ART because they ‘were taking another medication’ (17.2% vs 6.0% respectively; p<0.001). Similarly, those reporting ‘I must take food at the same time as my HIV treatment’, had three-fold higher prevalence than those not on ART with food requirements, to miss ART dose because they had ‘a problem taking pills at specified times (with meals, on empty stomach etc.)’ (22.5% vs 7.5% respectively; p<0.001). PLHIV on regimens containing integrase strand transfer inhibitors were less likely to report that ‘I must take food at the same time as my HIV treatment’, compared to those on regimens without integrase strand transfer inhibitors (35.0% [119/340] vs 53.2% [126/237], respectively; p<0.001). Conversely, reported food requirements with ART were significantly higher among those on regimens containing versus not containing protease inhibitors (50.8% [62/122] vs 40.2% [183/455]; p=0.035), or non-nucleoside reverse transcriptase inhibitors (55.9% [114/204] vs 35.1% [131/373], respectively; p<0.001). The percentage reporting ‘I cannot take another drug at the same time as my HIV treatment’ was significantly higher among those on regimens containing versus not containing protease inhibitors (24.6% [30/122] vs 13.8% [63/455], respectively; p=0.004), or nonnucleoside reverse transcriptase inhibitors (20.6% [42/204] vs 13.7% [51/373], respectively; p=0.031) (Table 3).
Table 3
Characteristics of people living with HIV on antiretroviral therapy in four European countries as well as the percentage reporting various medical conditions interfering with daily oral ART dosing and other treatment challenges, 2019
| Characteristics | Need monitoring when taking other medications with ART | Ever changed ART because of DDI | Unable to take antacids with ART | ART has food requirements | Cannot take another drug at the same time with ART | Ever hid or disguised ART | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | p | % | p | % | p | % | p | % | p | % | p | |
| Total | 19.1 | 15.4 | 23.9 | 42.5 | 16.1 | 43.3 | ||||||
| Gender | ||||||||||||
| Women | 27.1 | χ2(2)=11.51 | 24.7 | χ2(2)=16.12 | 29.4 | χ2(2)=4.52 | 54.1 | χ2(2)=16.61 | 21.8 | χ2(2)=5.97 | 39.7 | χ2(2)=1.81 |
| Men | 15.6 | p=0.003 | 11.6 | p<0.001 | 21.7 | p=0.105 | 37.3 | p<0.001 | 13.8 | p=0.051 | 45.1 | p=0.405 |
| Other | ¶ | ¶ | ¶ | ¶ | ¶ | ¶ | ||||||
| Sexual orientation | ||||||||||||
| Heterosexual | 24.9 | χ2(2)=6.52 | 21.3 | χ2(2)=9.81 | 26.6 | χ2(2)=1.01 | 53.3 | χ2(2)=12.22 | 17.2 | χ2(2)=5.87 | 39.5 | χ2(2)=3.30 |
| Homosexual | 16 | p=0.038 | 12.0 | p=0.007 | 22.9 | p=0.602 | 37.3 | p=0.002 | 14.4 | p=0.053 | 46.0 | p=0.192 |
| Other | 24.2 | 24.2 | 21.2 | 45.5 | 30.3 | 36.8 | ||||||
| Age (years) | ||||||||||||
| <50 | 20.4 | χ2(1)=1.75 | 16.5 | χ2(1)=1.37 | 26.8 | χ2(1)=6.36 | 44.3 | χ2(1)=1.94 | 19.0 | χ2(1)=8.64 | 50.2 | χ2(1)=31.58 |
| ≥50 | 15.7 | p=0.186 | 12.7 | p=0.241 | 16.9 | p=0.012 | 38.0 | p=0.164 | 9.0 | p=0.003 | 27.0 | p<0.001 |
| Education level | ||||||||||||
| Postgraduate | 23.3 | χ2(3)=5.21 | 13.3 | χ2(3)=1.87 | 31.7 | χ2(3)=7.68 | 43.3 | χ2(3)=1.19 | 20.0 | χ2(3)=6.29 | 64.2 | χ2(3)=30.15 |
| College | 19.6 | p=0.157 | 16.6 | p=0.600 | 23.9 | p=0.053 | 43.9 | p=0.756 | 14.4 | p=0.098 | 40.1 | p<0.001 |
| Secondary | 12.3 | 12.3 | 17.3 | 43.2 | 21.0 | 34.3 | ||||||
| Other | 11.4 | 20.0 | 14.3 | 34.3 | 5.7 | 34.1 | ||||||
| Employment | ||||||||||||
| Employed | 18.3 | χ2(1)=0.23 | 14.9 | χ2(1)=0.34 | 25.5 | χ2(1)=1.31 | 43.0 | χ2(1)<0.01 | 15.6 | χ2(1)=0.11 | 48.6 | χ2(1)=14.25 |
| Non-employed | 20 | p=0.629 | 16.8 | p=0.558 | 21.1 | p=0.253 | 43.2 | p=0.951 | 16.8 | p=0.737 | 33.0 | p<0.001 |
| Country | ||||||||||||
| France | 30.4 | χ2(3)=23.06 | 28.9 | χ2(3)=24.56 | 36.3 | χ2(3)=29.52 | 56.3 | χ2(3)=14.40 | 18.5 | χ2(3)=5.13 | 46.5 | χ2(3)=23.09 |
| Germany | 8.2 | p<0.001 | 10.9 | p<0.001 | 9.5 | p<0.001 | 37.4 | p=0.002 | 10.2 | p=0.163 | 29.8 | p<0.001 |
| Italy | 21.3 | 11.0 | 28.3 | 36.2 | 18.1 | 54.0 | ||||||
| UK | 17.9 | 11.9 | 23.2 | 40.5 | 17.9 | 46.4 | ||||||
| ART formulation | ||||||||||||
| Single table | 18.2 | χ2(1)=0.36 | 13.5 | χ2(1)=2.07 | 24.8 | χ2(1)=0.28 | 47.0 | χ2(1)=6.07 | 16.3 | χ2(1)=0.02 | 45.7 | χ2(1)=1.93 |
| Multi-tablet | 20.2 | p=0.549 | 17.8 | p=0.150 | 22.9 | p=0.595 | 36.8 | p=0.014 | 15.9 | p=0.894 | 40.4 | p=0.165 |
| Self-reported viral status | ||||||||||||
| Non-suppressed | 31.2 | χ2(1)=6.93 | 34.4 | χ2(1)=19.82 | 42.2 | χ2(1)=13.20 | 53.1 | χ2(1)=3.35 | 34.4 | χ2(1)=17.75 | 54.8 | χ2(1)=4.38 |
| Suppressed | 17.5 | p=0.008 | 13.1 | p<0.001 | 21.6 | p<0.001 | 41.1 | p=0.067 | 13.8 | p<0.001 | 42.0 | p=0.036 |
| ART side effectsa | ||||||||||||
| None | 14.4 | χ2(2)=13.50 | 10.7 | χ2(2)=23.56 | 18.5 | χ2(2)=8.77 | 34.7 | χ2(2)=15.55 | 11.4 | χ2(2)=13.10 | 38.7 | χ2(2)=12.00 |
| Non-gastrointestinal only | 13.8 | p=0.001 | 6.2 | p<0.001 | 26.2 | p=0.012 | 41.2 | p<0.001 | 12.5 | p=0.001 | 37.5 | p=0.002 |
| Gastrointestinal | 26.5 | 24.3 | 29.6 | 52.2 | 23.0 | 52.0 | ||||||
| HIV diagnosis year | ||||||||||||
| 2017–19 | 29.4 | χ2(2)=4.37 | 7.8 | χ2(2)=3.48 | 33.3 | χ2(2)=8.78 | 56.9 | χ2(2)=7.37 | 19.6 | χ2(2)=7.54 | 37.5 | χ2(2)=27.01 |
| 2010–16 | 19.2 | p=0.113 | 17.7 | p=0.175 | 27.5 | p=0.012 | 44.5 | p=0.025 | 20.0 | p=0.023 | 54.9 | p<0.001 |
| Pre-2010 | 16.9 | 14.6 | 18.4 | 37.5 | 11.5 | 34.4 | ||||||
| NNRTI-containing ARTb | ||||||||||||
| No | 16.6 | χ2(1)=4.08 | 14.2 | χ2(1)=1.19 | 20.4 | χ2(1)=7.27 | 35.1 | χ2(1)=23.27 | 13.7 | χ2(1)=4.66 | 40.9 | χ2(1)=3.12 |
| Yes | 23.5 | p=0.043 | 17.6 | p=0.274 | 30.4 | p=0.007 | 55.9 | p<0.001 | 20.6 | p=0.031 | 47.9 | p=0.078 |
| Entry inhibitorcontaining ARTc | ||||||||||||
| No | 18.5 | χ2(1)=3.41 | 14.0 | χ2(1)=24.87 | 23.7 | χ2(1)=0.42 | 42.2 | χ2(1)=0.48 | 16.0 | χ2(1)=0.23 | 43.4 | (1)=0.08 |
| Yes | 35 | p=0.065 | 55.0 | p<0.001 | 30.0 | p=0.516 | 50.0 | p=0.488 | 20.0 | p=0.631 | 40.7 | p=0.783 |
| INSTI-containing ARTd | ||||||||||||
| No | 19.8 | χ2(1)=0.15 | 14.3 | χ2(1)=0.36 | 23.6 | χ2(1)=0.02 | 53.2 | χ2(1)=18.86 | 16.0 | χ2(1)<0.01 | 45.0 | χ2(1)=0.62 |
| Yes | 18.5 | p=0.695 | 16.2 | 0.549 | 24.1 | p=0.892 | 35.0 | p<0.001 | 16.2 | p=0.963 | 42.0 | p=0.433 |
| Protease inhibitorcontaining ARTe | ||||||||||||
| No | 17.4 | χ2(1)=4.04 | 14.3 | χ2(1)=2.14 | 23.7 | χ2(1) =0.04 | 40.2 | χ2(1)=4.42 | 13.8 | χ2(1)=8.21 | 43.3 | χ2(1)<0.01 |
| Yes | 25.4 | p=0.044 | 19.7 | p=0.144 | 24.6 | p=0.844 | 50.8 | p=0.035 | 24.6 | p=0.004 | 43.3 | p=0.996 |
| Past ART resistancef | ||||||||||||
| No | 18.1 | χ2(1)=2.63 | 13.1 | χ2(1)=16.57 | 23.0 | χ2(1)=1.78 | 41.7 | χ2(1)=1.03 | 14.7 | χ2(1)=6.07 | 42.6 | χ2(1)=0.96 |
| Yes | 26 | p=0.105 | 31.5 | p<0.001 | 30.1 | p=0.183 | 47.9 | p=0.310 | 26.0 | p=0.014 | 48.2 | p=0.328 |
ART: antiretroviral therapy (the different classes presented in table are not mutually exclusive). NNRTI: non-nucleoside reverse transcriptase inhibitor. INSTI: integrase strand transfer inhibitor.
a A history of major ART side effects was said to be present if the respondent reported a past adverse effect from HIV medication (e.g. ‘stomach/gastric problems because of the medication’ or ‘difficulties taking my HIV treatment as I was having too many side effects’), that led to stopping ART, switching ART, or failing to achieve viral suppression from poor adherence, all because of the side effects.
b NNRTI-containing regimens included ‘Atripla® or generics (emtricitabine/efavirenz/tenofovir disoproxil fumarate)’; ‘Delstrigo (doravirine/lamivudine/tenofovir disoproxil fumarate)’; ‘Edurant (rilpivirine)’; ‘Eviplera (emtricitabine/rilpivirine/tenofovir -disoproxil fumarate)’; ‘Viramune or generics (Nevirapin)’; ‘Sustiva or generics (efavirenz)’; ‘Odefsey (emtricitabine/ rilpivirine/tenofovir alafenamide)’; or ‘Pifeltro (doravirine)’.
c Entry inhibitor-containing regimens included ‘Celsentri (maraviroc)’; ‘Fuzeon (enfuvirtide)’ or ‘Fostemsavir’.
d INSTI-containing regimens included ‘Genvoya (elvitegravir/cobicistat/ emtricitabine/tenofovir alafenamide)’; ‘Tivicay (dolutegravir)’; ‘Triumeq (dolutegravir/abacavir/lamivudine)’; ‘Isentress (raltegravir)’; ‘Juluca (dolutegravir/rilpivirine)’; ‘Stribild (elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate); or ‘Biktarvy (bictegravir/emtricitabine/tenofovir alafenamide)’.
e Protease inhibitor-containing regimens included ‘Kaletra (lopinavir/ritonavir)’; ‘Evotaz (atazanavir/cobicistat)’; ‘Prezista (darunavir)’; ‘Reyataz (atazanavir)’; ‘Rezolsta (darunavir/ cobicistat)’; or ‘Symtuza (darunavir/emtricitabine/tenofovir alafenamide)’.
f A history of resistance to ART was said to be present if this was reported as the reason for the respondent having stopped ART, switched ART, or failed to achieve viral suppression; or if the respondent was currently on Fuzeon (enfuvirtide), whose main indication is for treatment-experienced patients with evidence of HIV-1 replication despite ongoing ART.
Figure 1
A comparison of HCP and PLHIV-reported prevalence estimates for various medical, emotional, and psychosocial challenges to daily oral administration of HIV medicines among adults living with HIV on antiretroviral therapy in four European countries, 2019
Daily oral ART and challenges with adherence
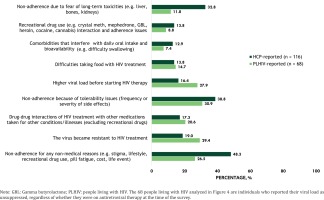
Of PLHIV on ART, 23.8% [164/688] reported suboptimal adherence to daily oral ART (Figure 1); the corresponding estimate reported by HCPs was 33.6%. Furthermore, 40.1% [276/688] of PLHIV reported being worried about missing their daily oral ART. PLHIV-reported average time (minutes) spent on daily oral ART administration was as follows: overall, 12.3 (SD=32.3); with malabsorption, 25.7 (SD=52.4); with interfering gastrointestinal conditions interfering, 24.9 (SD=47.7); with difficulty swallowing, 33.1 (SD=55.7); with neurocognitive conditions, 12.6 (SD=29.0); and with none of these four conditions, 6.3 (SD=16.6) minutes. The percentage of PLHIV needing help from someone to take their daily oral ART was as follows: with malabsorption, 17.0% (9/53); with interfering gastrointestinal condition, 14.8% (19/128); and with neurocognitive conditions, 16.1% (38/236). The percentage spending more time or effort to take their daily oral ART because of their condition was: with malabsorption, 30.2% (16/53); with interfering gastrointestinal condition, 35.2% (45/128); and with neurocognitive conditions, 33.9% (80/236). According to HCPs, patients ‘not taking HIV medication as prescribed (non-adherent) for any nonmedical reasons (e.g. stigma, lifestyle, recreational drug use, pill fatigue, cost, life event)’, was the leading reason ‘for not being suppressed’ (48.3%) (Figure 4). Other perceived reasons ‘for not being suppressed’, from an HCP perspective, included patient ‘not taking HIV medication as prescribed (non-adherent) because of tolerability issues’ (38.8%), ‘patient non-adherence due to fear of long-term toxicities’ (32.8%), or because ‘patient's virus became resistant to HIV treatment’ (19.0%). The top reasons reported by PLHIV who were virally non-suppressed (n=68) included ‘difficulties taking my HIV treatment as I was having too many side effects’ (30.9%; 21/68), ‘the virus became resistant’ (29.4%; 20/68), ‘higher viral load before starting HIV therapy’ (27.9%; 19/68), and ‘difficulties taking my HIV pill (s) every day for any reason except another medical condition’ (26.5%; 18/68).
Confidentiality concerns associated with daily oral ART
Among PLHIV, openness in sharing HIV status varied, from 29.7% (204/688) who reported that ‘no one knows about my HIV status’; to 20.9% (144/688) who indicated ‘I've limited what I tell others about my HIV’, to 7.6% (52/688) who reported being ‘generally open in talking about my status’. The percentage of PLHIV who reported they had shared their HIV status within various relationships was as follows, as applicable: with a partner/spouse/significant-other (91.0%; 424/466), with other sexual partners (75.6%; 391/517), with parents/siblings/children (78.0%; 478/613), with wider family members (55.0%; 333/605), with close friends (85.4%; 534/625), with current family doctor (89.8%; 561/625), with other healthcare professionals such as nurses, counsellors, pharmacists, and psychiatrists (84.0%; 516/614), with current employer (33.4%; 167/500), and with co-workers at current workplace (37.5%; 190/507). To avoid sharing their HIV status, 43.3% (298/688) reported hiding their HIV medications at least once within the past 6 months; over half of those who hid their medication (53.7%; 160/298) admitted they would feel stressed/anxious ‘if someone [they] did not want to see [their] HIV pills were to find them’. Overall, 16.1% (111/688) reported that ‘keeping my pills at home or with me during the day, I have been worried someone would see them and know about my HIV’. Specific situations where PLHIV were worried about unwanted disclosure of their HIV status included: while travelling at airports, by airport security or customs (22.2%; 153/688); while on holiday, by friends (15.1%; 104/688); while at the workplace, by coworkers (17.2%; 118/688); or while at home, by family members (10.2%; 70/688). One reason reported by PLHIV for not wanting to share their HIV status was worry ‘that I'll lose my source of income if other people find out that I have HIV’ (16.7%; 115/688). Overall, 16.6% (114/688) reported having missed ART doses because they ‘were not in a situation where [they] felt comfortable taking [their] pills (privacy/confidentiality)’; 40.0% (275/688) felt that ‘taking my HIV treatment less often (for instance every 2 months instead of every day) would reduce the shame or stigma I feel for having HIV’.
Emotional wellbeing related to daily tablet requirements
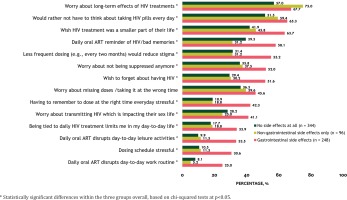
Overall, 45.1% (310/688) of PLHIV felt that ‘taking daily HIV treatment reminds me that I have HIV and/or of a mistake or bad memory from my past’, and 50.3% (346/688) wished ‘my HIV treatment was a smaller part of my life’. Many felt that ‘being tied to my daily HIV treatment limits me in my day-to-day life’ (23.7%; 163/688). Furthermore, 27.3% (188/688) felt that ‘having to remember to take my HIV treatment at the right time every day causes me stress or anxiety’; 57.6% (396/688) ‘would rather not have to think about taking the pills every day’; 41.9% (288/688) reported ‘worry about missing doses and not being suppressed anymore’; while 32.4% (223/688) indicated ‘I worry about missing doses and transmitting the disease which is impacting my sex life’. These concerns were particularly pronounced among those experiencing gastrointestinal side effects from their ART (Figure 5). Differences existed on some measures between PLHIV and HCP estimates. For example, 63.4% (436/688) of PLHIV reported being worried about long-term effects of HIV treatment, almost twice the estimate reported by HCPs in relation to HIV patients (33.3%) (Figure 1).
DISCUSSION
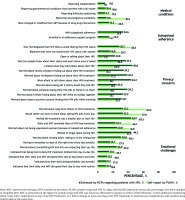
We found that a substantial proportion of PLHIV reported several problems related to daily oral dosing, including medical conditions that limit oral administration, emotional challenges (e.g. pill fatigue), privacy and confidentiality concerns, all of which collectively contribute to poor adherence. While HCPs were also aware of the confidentiality and emotional concerns faced by patients, HCPs’ estimates of these issues were lower than those reported by PLHIV. This suggests that not all patients discuss HIV-related emotional challenges with their HCPs27. The gap between perceived and actual emotional wellbeing among PLHIV underscores the urgent need to address the stigma still surrounding HIV to improve quality of life32-34. Such stigma may explain findings from previous studies showing that on dimensions of mental health and psychological factors, PLHIV show a detriment versus the general population35. Consistent with our findings, previous research has documented patients’ worries that their HIV status may be disclosed inadvertently if they store their antiretroviral medications at home and take them at regular intervals; therefore, patients have reported skipping or delaying doses to avoid stigma12.
The percentage of PLHIV with suboptimal adherence in our study (23.8%) is very close to the 24.1% overall estimate reported in a recent multi-country study12. Another study by Okoli et al.36 found that 66.6% of PLHIV worried about the long-term effects of HIV medicines, similar to 63.4% in our study. That same study reported that 18.6% (288/1550) of those who ever switched ART (or 13.6% [288/2112] of all study participants) reported switching because of drug-drug interactions; in our study, 15.4% of all participants reported ever changing ART because of drug-drug interactions. Regarding confidentiality concerns, our finding that only 7.6% of participants reported being fully open in discussing their HIV status is similar to the estimate of 6.8% reported in the 2019 Positive Perspectives Survey from 25 countries regarding the percentage of PLHIV who ‘always’ shared their HIV status37. The population subgroups with the highest prevalence of swallowing difficulty in our study included women, younger adults, those on multi-tablet regimens, and those experiencing gastrointestinal side effects. Some of these subgroups have been identified in previous research as being at highest risk for suboptimal adherence12.
Current approaches to improving the ease of drug administration for patients with dysphagia or pill aversion include reducing the number of pills and altering the drug form38. The use of small pills has been effective for suppressing viral load in some patients with dysphagia39. Crushing pills or opening capsules may be acceptable in some cases; however, many antiretroviral agents are not recommended for crushing (if a pill) or opening (if a capsule) because the change in the formulation can reduce its bioavailability and targeted therapeutic exposure40. New non-oral long-acting directly observed ARTs could potentially address some of the unmet problems identified in this study. Indeed, previous studies show that many PLHIV believe that a long-acting ART would address some of the challenges they face with daily oral ART20,21.
Strengths and limitations
The strength of this study is that we have explored both PLHIV and HCP perspectives regarding challenges to daily oral dosing in four European countries. Self-reported HIV diagnosis was followed by a confirmed ascertainment of HIV status for all PLHIV. Sampling was done such that the distribution of participants on key demographic variables matched national statistics for all PLHIV. Nonetheless, limitations exist. First, the HCP and PLHIV data in each country may not be directly comparable as the institutions from which the HCPs were mostly sampled may not necessarily reflect places where sampled PLHIV routinely access care. Second, these are cross-sectional analyses and only associations can be drawn. Third, neither the HCP nor PLHIV data may be fully representative of the respective countries or region, because of the non-probabilistic sampling.
CONCLUSIONS
Many treatment-related problems were identified in relation to daily intake of oral medication among PLHIV. HCPs estimated that approximately 10–15% of their patients were affected by each medical condition identified as interfering with daily oral administration. HCPs identified ‘nonadherence for any non-medical reason’ as the primary cause of virologic failure. Of surveyed PLHIV, 43.3% reported hiding their medication, while 45.1% saw their tablets as a daily reminder of HIV. New non-oral long-acting directly observed ARTs could help address these problems and improve longterm outcomes among PLHIV.