INTRODUCTION
Tobacco use is a global epidemic that affects all levels of society and is one of the leading causes of preventable morbidity and mortality in the world. According to the data of the World Health Organization (WHO), the tobacco epidemic kills more than 8 million people a year1. According to the latest data, overall smoking prevalence is 31.6%; 44.1% among males and 19.2% among females in Türkiye2. Quitting tobacco becomes very important for smokers to improve their health, and nearly 70% of smokers want to quit; however, it can be challenging due to nicotine addiction. Additionally, only fewer than a third of smokers who try to quit use evidence-based cessation methods3. Healthcare providers play a key role in not only encouraging smokers to quit but also providing evidence-based cessation methods available in smoking cessation outpatient clinics. In Türkiye, the number of smoking cessation clinics is increasing, the current number is around 4204. Due to the availability of free smoking cessation medications, admission rates to smoking cessation services are increasing; however, the effect on the quit rate is not much different according to published data from outpatient smoking cessation clinics5,6.
Evidence-based smoking cessation methods include a combination of cognitive behavioral support and pharmacotherapy. Among the medication choices, varenicline, nicotine replacement therapies and bupropion are recommended as the first choice7. In a meta-analysis, the combination of medication and behavioral counselling has been associated with a quit rate of 15.2% over 6 months compared with a quit rate of 8.6% with brief advice or usual care8. A randomized double-blind clinical trial of 8144 smokers, which directly compared the efficacy and safety of varenicline, bupropion, nicotine patch, and a placebo, has found a significantly higher 6-month quit rate for varenicline (21.8%) compared to bupropion (16.2%) and the nicotine patch (15.7%). Each therapy has been found more effective than a placebo (9.4%)9. In a recent study evaluating long-term abstinence rates of smokers in real-life setting has shown that long-term abstinence rate is 15.2% in varenicline users and 10.3% in NRT users10.
In a recent study examining the effectiveness of 448 SCCs in China, the quit rate at 3 months was found to be 23.5%. It indicates that interventions provided by SCCs can be effective. Patients with lower nicotine dependence, higher education level, and higher family income reported higher abstinence rates, which aligns with previous research. The same study also found that exhaled carbon monoxide (ECO) testing could improve abstinence rates. However, only 70.2% of SCCs were equipped with ECO detectors, and only 36.2% of outpatients were tested11.
It is known that access to evidence-based smoking cessation support is at a critical point in smoking cessation support and access to this support has become widespread through smoking cessation clinics. In a recent article examining the utilization of SCCs in Saudi Arabia, it was stated that limited information is available about the awareness of SCCs among smokers, the utilization of clinics, and the effectiveness of these clinics12. However, monitoring and monitoring outcomes are important for the functioning and effectiveness of these clinics. In order to identify and improve the deficiencies in the progress, we wanted to examine the SCC results of our hospital, which we first established. Thus, we aimed to evaluate the quit status of individuals applying to smoking cessation outpatient clinics in long-term follow-up and the associated factors. From this point of view, our work will be valuable as it provides a comprehensive overview of real-life applications regarding smoking cessation interventions.
METHODS
Study design and setting
This retrospective cohort study was conducted at the smoking cessation outpatient clinic of the pulmonology department of a tertiary care university affiliated education and research government hospital after having received institutional ethics committee approval dated 26.11.2020 and numbered 2020/23. The smoking cessation outpatient clinic serves within the Chest Diseases Department and gives outpatient services provided by a pulmonologist, who has a Smoking Cessation Advisory Certificate by the Ministry of Health (MoH), and a medical secretary, once a week. All patients in that study were evaluated by and consulted to the same physician (DK). In the clinic, free varenicline and nicotine patches were distributed by MoH and applications can be made by appointment.
Study protocol
At first admission, the patients were evaluated with their past medical histories, physical examinations, laboratory tests, chest X-ray, pulmonary function test, and electrocardiography. Patients with psychiatric and cardiac comorbidities were referred to the relevant departments. Data were recorded on online Tobacco Addiction Treatment Monitoring System (TUBATIS) and manually to previously created files. Detailed steps of the online system have been described in previous studies5,13. At the final step of TUBATIS system, FTND scores14 were automatically calculated, and the patients were informed about the planned treatment. Also written informed consent form that explains the potential side effects of the smoking cessation pharmacotherapy choices was obtained from the patients. The target quit date and the date for the next appointment were also recorded. Patients who applied to the smoking cessation outpatient clinic were given free of charge varenicline (Champix 0.5 mg and 1 mg) and/or nicotine patches (nicorette transdermal plaster 25 mg as step 1 and 15 mg as step 2). The system is set to give enough medication for a maximum of 4 weeks in each assignment and 12 weeks of treatment is provided free of charge for each individual. The preference of medication was mainly based on patients’ choice and the availability of the medication in stock. The patients were given an appointment within two weeks, particularly at their target quit date, and adverse reactions and smoking cessation status were noted. Afterwards, patients were given an appointment for the 4th week of the first medication assigned, and upcoming appointments were made for the 8th and 12th weeks.
For this study protocol, in the second year of their admissions, the patients were called by phone and their smoking status was inquired. During follow-up visits, quit status was based on self-reports.
The national quitline (ALO 171) calls the patients at least 7 times a year, including the target quit date, 1st week, 1st month, 2nd month, 3rd month, 6th month and the end of the 12th month, determined in the quit plan. With these callbacks, the General Directorate of Public Health monitors the quit status of individuals and encourages them to cope with nicotine withdrawal symptoms experienced during the smoking cessation process, thereby increasing their motivation15.
Inclusion and exclusion criteria
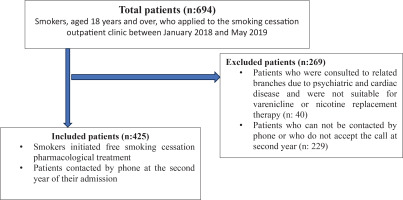
Included were smokers aged 18 and over who applied to our SCC between January 2018 and May 2019, who initiated at least one smoking cessation medication (NRT and/or varenicline) and were reached during the 2nd year phone calls. Smokers with mental disorders were excluded. Figure 1 shows the flow chart of selection of study participants, with inclusion and exclusion criteria.
Data collection process
The data collection process was carried out by SA and DK. The data available in the TUBATIS system was also available in manually prepared forms. The ID and telephone information of the patients were only available to these two researchers, and no variables that would reveal the ID were included during the transfer to the database.
Demographic characteristics, age, sex, occupation, and education level were recorded. Smoking status, age at which they started smoking, FTND scores, and previous smoking cessation attempts were recorded. Smoking cessation treatment initiated at SCC admission was recorded. The information recorded in follow-up applications were as follows: duration of use of the initiated treatment, and side effects experienced due to the treatment. Questions were asked in the 2nd year by phone. Those who stopped smoking after the target quit date were defined as quitters, the rest as non-quitters. Participants were also asked about the number of days they used the smoking cessation treatment, the number of times they attended the control SCC visit, and the side effects of the medication. Patients who had used smoking cessation medications for a month or less were classified as non-adherent to treatment.
Statistical and power analyses
The sample size of the study was calculated using G*Power 3.1.9.7 software. When the effect size is 0.25, α=0.05 and power is 0.99, the minimum number of participants to be reached is calculated as 278. Even so, our study included all patients who applied within the targeted time frame. When the smoking cessation prevalence is evaluated as 25%, the calculated effect size is 0.5. For the parameters we analyze for power, an effect size value of 0.30 is considered medium. In our research, we chose the value of 0.25, which is a lower step, because it is reasonable to use an effect size that will give stronger results than a medium value.
Processing and statistical evaluations of all data in our study were carried out using IBM SPSS Statistics 25.0 (SPSS, Inc, Chicago, IL, USA) package program. Differences between the groups were determined by Student’s t-test or Mann Whitney U test in numeric variables, and the relations between categorical variables were determined by chi-squared analysis. The associated factors on treatment adherence and quit success were analyzed by multivariable binary logistic regression analysis. Statistically significantly different variables present in the univariate analysis were used in multivariable models. Results are presented as odds ratios and 95% CIs. A p-value <0.05 was considered significant, all tests were 2-tailed.
RESULTS
A total of 425 patients were evaluated, the mean age was 38 ± 12 years, and 73.4% of them were males. The majority of the patients initiated varenicline (48%) and NRT (46.6%), and during the treatment period, 48% of them reported nicotine withdrawal symptoms. At the second year of the follow-up period, 20.5% of the sample quit successfully. According to age groups, quit rates were found to be 36.4% in those aged ≥65 years, 19.6% in those aged 45–64 years, 23.6% in the 25–44 years age range, and 6.6% in those aged <25 years.
Table 1 shows the characteristics of the study population and differences according to sex and statistically significant differences were as follow: While the majority of women had a primary school education level (53%), the majority of men had an education level of high school or above (68.5%); the smoking initiation age of men was lower than that of women; the majority of smoker women were employed (66.3%), while the majority of men were unemployed (71.7%); and the presence of past history of psychiatric illness was at a higher rate in women than in men (p=0.001).
Table 1
Characteristics of the study population according to gender, smoking cessation outpatient clinic, Recep Tayyip Erdoğan University Training and Research Hospital, January 2018 and May 2019 (N=425)
| Characteristics | All(N=425) n (%) | Male(N=312) n (%) | Female(N=113) n (%) | p |
|---|---|---|---|---|
| Age, mean (SD) | 38.48 (12.04) | 38.17 (12.53) | 39.35 (10.61) | 0.376* |
| Age (years) | 0.352** | |||
| <25 | 61 (14.4) | 50 (16.0) | 11 (9.7) | |
| 25–44 | 246 (57.9) | 177 (56.7) | 69 (61.0) | |
| 45–64 | 107 (25.2) | 76 (24.3) | 31 (27.4) | |
| ≥65 | 11 (2.6) | 9 (2.8) | 2 (1.7) | |
| Education level | <0.001** | |||
| Primary school | 158 (37.2) | 98 (31.4) | 60 (53.0) | |
| High school | 143 (33.6) | 110 (35.2) | 33 (29.2) | |
| University | 124 (29.2) | 104 (33.3) | 20 (17.6) | |
| Smoking initiation age, median (IQR) (range) | 17.3 (5) (8–34) | 17 (3) | 18 (4) | <0.001*** |
| Smoking pack-years, median (IQR) | 18 (18) | 18.50 (20) | 20 (20) | 0.196*** |
| Fagerström test score, median (IQR) | 6 (4) | 6 (4) | 7 (3) | 0.776*** |
| Employment status | <0.001** | |||
| Employed | 258 (60.7) | 43 (13.7) | 75 (66.3) | |
| Unemployed | 118 (27.8) | 224 (71.7) | 34 (30.0) | |
| Student | 49 (11.5) | 45 (14.4) | 4 (3.5) | |
| Comorbidities | ||||
| Cardiovascular disease | 68 | 55 (17.6) | 13 (11.5) | 0.128** |
| Pulmonary disease | 53 | 40 (12.8) | 13 (11.5) | 0.717** |
| Psychiatric illness | 39 | 20 (6.4) | 19 (16.8) | 0.001** |
| Previous quit attempt, median (IQR) | 2 (3) | 2 (3) | 2 (2) | 0.568*** |
| Abstinence days in previous quit attempts, median (IQR) | 30 (120) | 30 (120) | 16.5 (173) | 0.708*** |
| Initiated smoking cessation medication | 0.059** | |||
| Varenicline | 204 (48.0) | 141 (45.1) | 63 (55.7) | |
| Nicotine replacement therapy | 198 (46.6) | 156 (50.0) | 42 (37.1) | |
| Combination of both | 23 (5.4) | 15 (4.8) | 8 (7.0) | |
| Days of medication use, median (IQR) | 60 (30) | 30 (30) | 30 (30) | 0.904*** |
| Adverse reactions | ||||
| Nicotine withdrawal | 204 (48.0) | 149 (47.7) | 55 (48.6) | 0.867** |
| Nausea/vomit/headache | 107 (25.1) | 72 (23.0) | 35 (30.9) | 0.098** |
| Sleep disorders/nightmares | 32 (7.5) | 25 (8.0) | 7 (6.1) | 0.530** |
| Weight gain/constipation | 99 (23.2) | 67 (21.4) | 32 (28.3) | 0.140** |
| Number of attended clinical visits, median (IQR) | 2 (1) | 1 (1) | 1.5 (1) | 0.638*** |
| Smoking cessation status | 0.334** | |||
| Quit successfully | 87 (20.5) | 55 (17.6) | 32 (28.3) | |
| Non-quitter | 338 (79.5) | 257 (82.3) | 81 (71.6) |
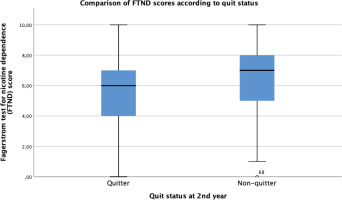
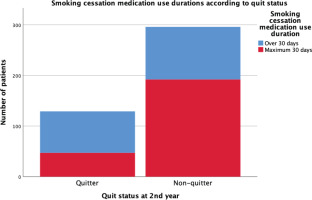
Figures 2 and 3 show the statistically significant differences of FTND scores of the sample according to quit status and treatment adherence status. Non-quitters and treatment non-adherent groups have higher nicotine dependence scores than the rest (p<0.05). Figure 4 shows the longer use duration of smoking cessation treatments of quitters than non-quitters (p<0.005).
Figure 2
Comparison of Fagerström test for nicotine dependence scores according to treatment adherence status
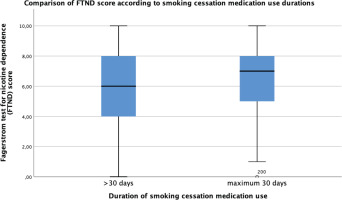
Table 2 shows the factors associated with treatment adherence in univariate and multivariate analyses. In multivariate analysis, FTND score (OR=1.14; 95% CI: 1.01–1.30) and unsuccessful quitting (OR=7.71; 95% CI: 3.44–17.31) were positively associated with non-adherence, whereas age (OR=0.97; 95% CI: 0.94–1.00), presence of nicotine withdrawal symptoms (OR=0.21; 95% CI: 0.11–0.39) and presence of weight gain/appetite increase/constipation (OR=0.35; 95% CI: 0.17–0.69) were negatively associated with non-adherence.
Table 2
Factors associated with non-adherence to smoking cessation treatment, smoking cessation outpatient clinic, Recep Tayyip Erdoğan University Training and Research Hospital, January 2018 and May 2019 (N=425)
| Variables | Univariable analysis* | Multivariable analysis | |||
|---|---|---|---|---|---|
| Non-adherent (N=239) n (%) | Adherent (N=186) n (%) | OR (95% CI) | AOR (95% CI) | p | |
| Age (years), mean (SD)† (per 1 age increase)§ | 36.4 (11.3) | 41.1 (12.4) | - | 0.970 (0.941–1.000) | 0.049 |
| Fagerström test score, median (IQR)† (per 1 score increase)§ | 7 (3) | 6 (4) | - | 1.143 (1.008–1.295) | 0.037 |
| Presence of withdrawal symptoms during the cessation period† (presence, Ref: absence)§ | 93 (45.6) | 111 (54.4) | 0.430 (0.291–0.637) | 0.210 (0.114–0.387) | <0.001 |
| Adverse reaction – weight gain/appetite increase /constipation† (presence, Ref: absence)§ | 31 (31.3) | 68 (68.7) | 0.259 (0.160–0.418) | 0.346 (0.173–0.693) | 0.003 |
| Rate of non-quitters† (non-quitter, Ref: quitter)§ | 219 (64.8) | 119 (35.2) | 3.221 (2.094–4.955) | 7.712 (3.437–17.305) | <0.001 |
| Duration of abstinence days in previous quit attempts, median (IQR)† (per 1 day increase)§ | 12,5 (90) | 60 (180) | - | 1.000 (0.999–1.001) | 0.585 |
| Presence of pulmonary disease history† (presence, Ref: absence)§ | 23 (43.4) | 30 (56.6) | 0.554 (0.330–0.990) | 0.851 (0.326–2.222) | 0.741 |
| Presence of adverse reaction nausea/vomiting/headache† (presence, Ref: absence)§ | 45 (42.1) | 62 (57.9) | 0.464 (0.297–0.724) | 0.559 (0.297–1.052) | 0.071 |
| Presence of adverse reaction sleep disorders/nightmares† (presence, Ref: absence)§ | 12 (37.5) | 20 (62.5) | 0.439 (0.209–0.923) | 0.479 (0.151–1.525) | 0.213 |
| Rate of NRT initiated smokers as pharmacological cessation method† (NRT users, Ref: varenicline users)§ | 133 (67.2) | 65 (32.8) | 2.336 (1.574–3.467) | 1.802 (0.980–3.314) | 0.058 |
| Rate of students compared to the other employment status† (student, Ref: other)§ | 35 (71.4) | 14 (28.6) | 2.108 (1.098–4.046) | 0.637 (0.224–1.809) | 0.397 |
AOR: adjusted odds ratio. Multivariate analysis model: Constant = -0.598. Enter test (likelihood ratio) = –2 Log likelihood = 311.996. Nagelkerke R2 = 0.396. Omnibus tests of model coefficient: p=0.000.
In multivariate logistic regression analysis, positively associated factors with failure in quitting during the 2 years were: being student compared to other employment status (AOR=6.82; 95% CI: 1.31–35.58), per 1 score increase in FTND score (AOR=1.16; 95% CI: 1.01–1.32), and presence of withdrawal symptoms during the smoking cessation treatment (AOR=7.09; 95% CI: 3.60–13.97). Negatively associated factors were; per 1-day increase in medication use duration (AOR=0.97; 95% CI: 0.95–1.00), presence of sleep disorders/nightmares (AOR=0.30; 95% CI: 0.11–0.79), and presence of weight gain/increased appetite/constipation (AOR=0.33; 95% CI: 0.18–0.63) (Table 3).
Table 3
Factors associated with unsuccessful quitting, smoking cessation outpatient clinic, Recep Tayyip Erdoğan University Training and Research Hospital, January 2018 and May 2019 (N=425)
| Variables | Univariable analysis* | Multivariable analysis | |||
|---|---|---|---|---|---|
| Quitter (N=87) n (%) | Non-quitter (N=338) n (%) | OR (95% CI) | AOR (95% CI) | p | |
| Employment status† (student, Ref: other)§ | 2 (4.1) | 47 (95.9) | 2.432 (1.106–5.347) | 6.818 (1.306–35.583) | 0.023 |
| Fagerström test score, median (IQR)† (per 1 score increase)§ | 6 (3) | 7 (3) | - | 1.157 (1.014–1.319) | 0.030 |
| Duration of treatment use, median (IQR)† (per 1 day increase)§ | 60 (0) | 30 (30) | - | 0.972 (0.947–0.999) | 0.040 |
| Experiencing sleep disorders/nightmares† (presence, Ref: absence)§ | 12 (37.5) | 20 (62.5) | 0.612 (0.293–1.280) | 0.301 (0.114–0.792) | 0.015 |
| Weight gain/appetite increase / constipation† (presence, Ref: absence)§ | 41 (41.4) | 58 (58.6) | 0.441 (0.276–0.703) | 0.334 (0.178–0.627) | 0.001 |
| Nicotine withdrawal symptoms† (presence, Ref: absence)§ | 26 (12.7) | 178 (87.3) | 2.630 (1.701–4.067) | 7.092 (3.601–13.967) | <0.001 |
| Number of visits to smoking cessation outpatient clinic, median (IQR)† (per 1 number increase)§ | 2 (0) | 1 (1) | - | 1.591 (0.858–2.950) | 0.141 |
| Smoking initiation age, median (IQR)† (per 1 age increase)§ | 18 (4) | 17 (3) | - | 0.942 (0.862–1.029) | 0.182 |
| Gender† (male, Ref: female)§ | 55 (17.6) | 257 (82.4) | 0.265 (0.168–0.417) | 0.549 (0.291–1.035) | 0.064 |
| Age (years), mean (SD)† (per 1 age increase)§ | 41.6 (11.5) | 37.6 (12.0) | - | 0.980 (0.954–1.007) | 0.149 |
DISCUSSION
In this study examining the long-term outcomes of our SCC, it was observed that the quit rate at the end of the 2nd year was 20.5%. Factors associated with the highest risk of not being able to quit smoking included being a student, experiencing withdrawal symptoms, and using treatment for short periods of time. FTND scores were also found to be higher in non-quitters and those who used the treatment for a shorter time than recommended.
The rate of successful quitters at outpatient smoking cessation clinics ranges 11–45% at six months to one year follow-up, depending on the characteristics of the smoker samples6,13,16-18. In our study, quit rates were found to be 36.4% in those aged ≥65 years, 19.6% in the 45–64 age range, 23.6% in the 25–44 age range, and 6.6% in those aged <25 years. While the rate of those aged ≥65 years in our sample was only 2.6%, the majority of the sample consisted of individuals younger than 45 years of age. The difference in our sample’s quit rate may be due to the high proportion of the younger age group. Among the factors of quit success, being a student increases the risk of not being able to quit. The influence of peers is an important factor and so tobacco-free university projects can propose solutions and should become widespread. Social environment, hobbies and sports facilities should be increased for students to avoid addictive behaviors like nicotine addiction19.
The presence of withdrawal symptoms was found to be positively associated with the inability to quit smoking. On the other hand, withdrawal symptoms can be managed by using smoking cessation treatments for a sufficient period of time as recommended. However, we found that there is a significant problem of compliance with these treatments. The majority of participants used these treatments for less than one month. Close monitoring and patient education should be increased in this regard; accordingly, it has been reported that the period of visits to the cessation clinic had been associated with quit success20. In our findings adherence to the standard smoking cessation treatment duration, which is 12 weeks, was insufficient7. In addition, 56.2% of the patients did not come to any follow-up visits after the first intervention. It was evaluated that besides patient-related factors such as age and nicotine dependence score, treatment side effects also had effects on treatment adherence. Supporting the management of side effects may be effective in increasing treatment compliance. Low adherence to follow-up outpatient clinic visits and lower treatment duration than the standard duration were the associated factors for our sample’s unsuccessful quitting attempts. In order to increase quit success, interventions to cover the gaps for smoking cessation clinics are required to increase both adherences.
Duration of treatment, presence of side effects experienced during the treatment process, as well as being a student and the FTND score were determined among the factors that also affect the success of smoking cessation. In various studies5, Metin girmek için buraya tıklayın veya dokunun. while older age positively affected the success of smoking cessation. Our study found that age was not statistically significant for cessation success, but we found treatment adherence increased in line with the age of the patients. However, regarding occupations, being a student was associated with failure to quit smoking. When evaluated by taking into account the existence of other employment status at a young age like students, it was shown that not only age but also social environment has an effect on quitting. This is in line with the theory of the more devastating effect of tobacco on socioeconomically disadvantaged groups. On the other hand, there is a positive correlation between adherence to treatment and smoking cessation and the increase in adherence to treatment as age increases, but the absence of only the age factor in achieving cessation success also supports this theory. In addition, students in Türkiye live in communal living areas like dormitories, and the presence of smoker friends also affects the possibility of relapse during cessation21.
In our study, the quit rate was not significantly different according to sex. In our sample, females were mostly primary school educated, and men were mostly high school or higher education level. Smoking initiation age of females was higher than men, the rate of being unemployed or a student was higher in males, and females were mostly employed. The rate of psychiatric illness was higher in women. It has been suggested that smoking women living in underdeveloped or developing countries may be more likely to have mental illness. This view has been suggested because women smoke and are more unsuccessful in quitting, although smoking by women is not socially accepted22.
Limitations
Although our study included a good sample size and presented findings that would be beneficial in clinical practice, obtaining smoking cessation status based on self-report is a limitation of the study. Exhaled carbon monoxide (ECO) levels of patients could not be measured due to financial situations within the scope of long-term success results. Due to the study design, other potential limitations are the non-causal inference and limited generalizability to other regions of the country or other countries.
Implications and future directions
According to reported publications, ECO test is effective in increasing patients’ willingness to quit smoking and improving quit rates. However, only 70.2% of SCCs were equipped with ECO detectors, and only 36.2% of outpatients underwent testing according to a novel study from China11. It is essential to provide and encourage the use of ECO detectors within SCCs to enhance testing rates and potentially improve patient outcomes. After this study, with the end of the COVID-19 pandemic, we were provided with the device and started to apply routine CO monitoring in our SCC23,24.
SCCs are effective services that provide access to evidence-based smoking cessation methods. However, the problem of compliance with follow-up visits and adequate treatment use continues. Improving these issues plays a vital role in increasing SCCs effectiveness. In order to keep the motivation of the applicants high, digital methods, such as reminder messages, can be tried and such digital health applications should be developed and tried in the field of smoking cessation support25.
CONCLUSIONS
In our sample, we determined the rate of successful quitters to be 20.5% in the long-term. The rate of using the treatment in the standard time was extremely low. Most of the patients did not come to control visits. In order to increase treatment compliance and control of the intervention, patients should be educated about treatment, and preliminary information and training should be given about coping with treatment side effects and withdrawal symptoms. In addition to the distribution of free medication, more intensive support and counselling services that increase motivation and adherence to smoking cessation treatments should be emphasized.